Abstract
Purpose
Materials and Methods
Results
Figures and Tables
Table 1
Characteristics of the Risk Factors for GB Polypoid Lesions (n=7134)
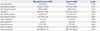
GB, gallbladder; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HP, Helicobacter pylori.
Data show mean±standard deviation, median (P25–P75), or absolute number (%). The level of significance is set below 0.05. Data show median (P25–P75) or absolute number (%). The level of significance is set below 0.05.
*Overweight status: body mass index over 23 kg/m2, †Data are presented as median (P25–P75) and analyzed using the Mann-Whiteny U test due to abnormal distribution, ‡Data show positive/esophagogastroduodenoscopy patients, §Data show positive/colonoscopy patients.
Table 2
Univariate and Multivariate Analyses of the Risk Factors for GB Polypoid Lesions (n=7134)

GB, gallbladder; OR, odds ratio; CI, confidence interval; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HP, Helicobacter pylori.
*Adjusted for LDL cholesterol, HDL cholesterol, chronic hepatitis B, and metabolic syndrome in logistic regression model, †Overweight status: body mass index over 23 kg/m2.
Table 3
Characteristics of the Risk Factors for GB Solitary Polypoid Lesions (n=2378)

GB, gallbladder; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HP, Helicobacter pylori.
Data show mean±standard deviation, median (P25–P75), or absolute number (%). The level of significance is set below 0.05.
*Overweight status: body mass index over 23 kg/m2, †Data are presented as median (P25–P75) and analyzed using the Mann-Whiteny U test due to abnormal distribution, ‡Data show positive/esophagogastroduodenoscopy patients, §Data show positive/colonoscopy patients.
Table 4
Univariate and Multivariate Analyses of the Risk Factors for GB Solitary Polypoid Lesions (n=2378)

GB, gallbladder; OR, odds ratio; CI, confidence interval; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HP, Helicobacter pylori.
*Adjusted for overweight status, HDL cholesterol, chronic hepatitis B, and metabolic syndrome in logistic regression model, †Overweight status: body mass index over 23 kg/m2.
Table 5
Characteristics of the Risk Factors for the Presence of Stones with GB Polypoid Lesions (n=2378)

GB, gallbladder; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HP, Helicobacter pylori.
Data show median (P25–P75) or absolute number (%). The level of significance is set below 0.05.
*Overweight status: body mass index over 23 kg/m2, †Data are presented as median (P25–P75) and analyzed using the Mann-Whiteny U test due to abnormal distribution, ‡Data show positive/esophagogastroduodenoscopy patients, §Data show positive/colonoscopy patients.
Table 6
Univariate and Multivariate Analyses of the Risk Factors for the Presence of Stones with GB Polypoid lesions (n=2378)

GB, gallbladder; OR, odds ratio; CI, confidence interval; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HP, Helicobacter pylori.
*Adjusted for overweight status, HDL cholesterol, metabolic syndrome, and gastric HP infection in logistic regression model, †Overweight status: body mass index over 23 kg/m2.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download