Abstract
Purpose
A phase I clinical trial was conducted to evaluate the immunogenicity and safety of newly developed egg-cultivated trivalent inactivated split influenza vaccine (TIV) in Korea.
Materials and Methods
The TIV was administered to 43 healthy male adults. Subjects with high pre-existing titers were excluded in a screening step. Immune response was measured by a hemagglutination inhibition (HI) assay.
Results
The seroprotection rates against A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2009 were 74.42% [95% confidence interval (CI): 61.38–87.46], 72.09% (95% CI: 58.69–85.50), and 86.05% (95% CI: 75.69–96.40), respectively. Calculated seroconversion rates were 74.42% (95% CI: 61.38–87.46), 74.42% (95% CI: 61.38–87.46), and 79.07% (95% CI: 66.91–91.23), respectively. There were 25 episodes of solicited local adverse events in 21 subjects (47.73%), 21 episodes of solicited general adverse events in 16 subjects (36.36%) and 5 episodes of unsolicited adverse events in 5 subjects (11.36%). All adverse events were grade 1 or 2 and disappeared within three days.
Influenza virus spreads with high infectivity via respiratory droplets and direct contact, resulting in winter outbreaks.1 Influenza may cause systemic symptoms and respiratory complications, particularly in vulnerable persons including children younger than 5 years, adults older than 65 years, and patients with chronic cardiopulmonary diseases.1 The World Health Organization (WHO) reported that three to five million cases of severe influenza occur annually with a mortality rate of 25–50%.1
Vaccination is necessary to control influenza, and the vaccination rate has risen steadily.23 Considering the increasing medical cost and economic burden of influenza treatment4 and the emergence of resistance against anti-viral agents,56 influenza vaccination is the most cost-effective method for controlling influenza.78 During the influenza pandemic of 2009, vaccine shortage occurred owing to the need to import raw material to manufacture the influenza vaccine. After this pandemic, chicken egg-based purified trivalent inactivated split influenza vaccine (TIV) was developed in Korea to supply safe and effective influenza vaccines through indigenous production.
Phase I clinical trial was conducted at Seoul St. Mary's Hospital from September 19 to October 29, 2012. Healthy male subjects aged 20–55 years with weights (≥55 kg) within 80 to 120% of ideal body weight were recruited for screening. Subjects with no congenital or chronic underlying disease, no acute illness during the past four weeks, and no abnormalities on physical examination, laboratory tests, chest X-ray, and electrocardiography were eligible for enrollment. Exclusion criteria included: acute or chronic illness, hematologic disease, previous history of Guillain-Barre syndrome, allergic disorders including egg allergy, immune-compromised state, long-term medication history or evidence of heavy inebriation 2–3 days before administering the vaccination. Those with pre-existing titers of 1:40 measured by a hemagglutination-inhibition (HI) assay were also excluded.
Enrolled subjects were prohibited from drinking alcohol from 1 day before to 3 days after the vaccination, and recommendations were made to avoid smoking, caffeine, and foods containing grapefruit extracts until 28 days after vaccination. All subjects were evaluated for safety on days 1, 2, 3, 14, and 28 after the vaccination. Immunogenicity was evaluated on day 28 after vaccination (final visit).
Purified TIV (egg cultivated, TIV, IL-YANG FLU Vaccine Vial INJ.) was administered in the deltoid muscle of the non-dominant arm. Each 0.5-mL dose of TIV contained 15 µg of hemagglutinin (HA) antigen prepared using Netherlands Vaccine Institute inactivation techniques. Antigens were A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2009, as recommended to the Northern Hemisphere by the WHO in 2011–2012.
Serum specimens were collected at baseline and 4 weeks after immunization. Approximately 5 mL of blood samples were collected, allowed to clot for about 30 minutes in a vertical position and then centrifuged. The serum samples were stored at -60℃ or below until laboratory assays. Antibody responses were measured by HI assay at a dilution of 1:10–1:1280 according to established procedures at the central laboratory, the Vaccine Bio Research Institute of the Catholic University of Korea, Seoul, Korea.1112 Guinea pig red blood cells and standard antigens from the National Institute for Biological Standards and Control (NIBSC) were used for HI assay: A/California/7/2009 (H1N1) NYMC X-179A (09/146), A/Perth/16/2009 (H3N2) (12/112), B/Brisbane/60/2009 (08/352).
The primary immunogenicity end point was seroprotection rate (SPR), or the percentage of subjects with a post vaccination titer of ≥1:40. The secondary end point was seroconversion rate (SCR), or the percentage of subjects with a pre-vaccination titer of <1:10 and a post vaccination titer of ≥1:40. The HI antibody responses were described by geometric mean titer (GMT) and geometric mean titer ratio (GMR). GMT is the anti-log of the arithmetic mean of log-10 transformed titers. The titers of anti-HA antibodies below the detection limit (i.e., <1:10) were assigned a value of 1:5.
All subjects were observed for 30 minutes for local and systemic reactogenicity and symptoms of immediate hypersensitivity following vaccination. Subjects received a diary card to record injection site reactions (pain, tenderness, redness, and swell-ing) and systemic adverse events up to day 7. All solicited and unsolicited local and systemic adverse events were recorded up to 28 days (days 8–28). The recorded events were classified according to MedDRA System Organ Class. Severity was recorded according to the U.S. Food and Drug Administration guidelines.13 All adverse events were assessed by investigators and reported to the Data Safety Monitoring Board (DSMB) committee, which assessed and confirmed the causality relationships of all solicited and unsolicited adverse events.
For secondary safety assessments, vital signs were checked and laboratory examinations (complete blood cell count, routine urinalysis, and blood chemistry) were conducted on the day of injection, day 14 and day 28 (final visit).
Safety analyses were performed at least once in all subjects who were vaccinated. The occurrence rates of solicited and unsolicited adverse events up to 28 days after vaccination were determined with 95% confidential intervals (CIs). They were analyzed according to age, history of disease, current medication, and use of long-term medication by using chi-square test or Fisher's exact test. Numerical values, such as vital signs and laboratory results, were measured before vaccination and 14 and 28 days after vaccination. The results were compared using paired t-test. The immunogenicity of the study vaccine was analyzed by comparing SPR, SCR, GMT, and GMR measured before and 28 days after vaccination.
Investigators explained the objective, process and expected results of this study to all enrolled subjects and received their informed consent. This study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice. The Ministry of Food and Drug Safety of Korea and the Institutional Board Review of the Catholic University of Korea Seoul St. Mary's Hospital (KC12BDSF0520) approved the protocol. The clinical trial was registered at http://www.clinicaltrials.gov (NCT02111070).
Among the 142 subjects who gave informed consent, 44 subjects were enrolled (98 screening failures). Ninety-one subjects were excluded because of a high pre-existing HI titer of 1:40 or more (Fig. 1). The study vaccine was administered to all enrolled subjects. Among the enrolled subjects, one subject drop-ped out owing to consumption of a prohibited drug (Fig. 1). The demographic and lifestyle characteristics of enrolled subjects are presented in Fig. 1.
SPRs (primary immunogenicity endpoints) against A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2009 met the CBER licensure criteria for influenza vaccines.14 Among the secondary immunogenicity endpoints, SCRs for all the strains exceeded 70%. Mean GMRs ranged from 5 to 9 (Table 1). As full analysis set was identical to the per-protocol set, immunogenicity results were also the same.
A total of 25 solicited local adverse events occurred in 21 subjects (47.73%), consisting of 13 episodes (29.55%) of pain, 11 episodes (25.00%) of tenderness, and one episode (2.27%) of swelling. Of the solicited local adverse events, there were 24 mild events, one moderate event, and no severe events. Twenty-one solicited general adverse events occurred in 16 subjects (36.64%), including fatigue in 11 subjects (25.00%), headache in 5 subjects (11.36%), myalgia in 2 subjects (4.55%), and nausea/vomiting, diarrhea, and fever in one subject (2.27%). Of the solicited general adverse events, there were 19 mild episodes and 2 moderate episodes; the latter consisted of fever and fatigue (Table 2). All of the solicited adverse events, regardless of severity and generality, disappeared within 3 days without any intervention.
The number of unsolicited adverse events was 5 in 5 subjects (11.36%). There was one event each of cough, sore throat, rhinorrhea, laceration, and transient amnesia. There were no severe unsolicited adverse events. The laceration and transient amnesia were of moderate severity, and no causal relationship between the adverse events and the study vaccine was found (Table 2).
Vital signs including body temperature, pulse rate, systolic blood pressure, and diastolic blood pressure were compared before vaccination, and on day 14, and 28 after vaccination. The pulse rates were significantly different, although all the results were within the normal range (Table 3). Laboratory tests results before vaccination and on days 14 and 28 after vaccination showed no significant differences. One subject showed elevated aspartate aminotransferase (83 U/L, normal range: 14–40 U/L), γ-glutamyl transpeptidase (109 U/L, normal range: 9–85 U/L), creatine phosphokinase (10130 U/L, normal range: 26–200 U/L), and lactate dehydrogenase (1095 U/L, normal range: 250–450 U/L) levels on day 14. All the elevated levels spontaneously normalized on day 28, and causal relationships between the study vaccine and laboratory abnormalities were suspected. One subject showed elevated γ-glutamyl transpeptidase level on day 14, which normalized on day 28. However, because his γ-glutamyl transpeptidase level before vaccination was also higher than normal, a causal relationship with the study vaccine was not acknowledged (Table 3). All other test results were within normal range.
In this clinical trial, an independent committee (DSMB) evaluated the severity of all noted adverse events. Relationships between the adverse events and the study vaccine were based on solicited and unsolicited adverse events, vital signs, and laboratory test results. The DSMB concluded that the study vaccine did not have any safety problems.
Although egg-cultivated inactivated influenza vaccine has been used since late 1930s, innate problems such as time constraints in the production cycle,15 reproducibility issues,16 and allergic reactions still remain. Mutation that may occur during viral growth in eggs has also been an issue.171819 Recently, cell-cultivated vaccines with efficacy and safety profiles comparable to those of egg-cultivated ones are available.202122 However, issues such as poor viral replication in Vero cells2324 or viral mutations252627 as in egg-based vaccines, adventitious viruses, residual cell line DNA protein,28 high cost for plant construction and maintenance2930 need to be resolved before they may replace the egg-cultivated one. As well as the cell-cultivated influenza vaccines based on the recombinant subunit, virus like particle or live-vectors are being developed.31323334353637 Their actual clinical efficacy, cost-effectiveness, and safety profiles in large populations through long-term use have not yet been established. Thus, chicken egg-cultivated inactivated vaccines which have verified effectiveness and trusted production processes, including selection of variants, manufacturing facilities, and regulatory oversight, still play an important role as seasonal influenza vaccines. 38
The vaccine studied in this report (IL-YANG FLU Vaccine Vial INJ.) is an egg-cultivated vaccine with reduced manufacturing time and improved sanitation: the eggs were decontaminated by fumigation with the large-scale vaporized hydrogen peroxide incubating system on the day of production (0–day old eggs). After incubation for 10 days, eggs were screened using a large-capacity candling machine that allows rapid and accurate inspection to exclude unfertilized or faulty eggs. Techniques applied to accelerate the zonal ultracentrifugation and splitting steps also contributed to the reduction of the manufacturing time.
Among the 142 subjects screened in this clinical study, 44 subjects were enrolled. All the 44 subjects were evaluated for safety and 43 subjects were evaluated for immunogenicity using the full analysis set (one subject dropped out of the study).
In the per-protocol set, SPRs against A/California/7/2009 (H1N1) and A/Perth/16/2009 (H3N2) were higher than 70%, but the lower limits of their 95% CIs were lower than 70% (Table 1). However, the significance of these findings is not conclusive because there were few enrolled subjects. All SCRs were above 70% with the lower limits of their 95% CIs above 60% (Table 1). Such high SCRs were attributed to the inclusion of subjects with antibody levels lower than 1:40 only. The subjects with high pre-existing HI titers were excluded based on a known negative correlation between high titer and conversion rates.1039 Several studies have reported that annual vaccination or high pre-vaccination titer is negatively correlated with antibody response,39404142 which differs among strains.43 It seems that the responsiveness is affected more by previous exposure to seasonal influenza vaccination than by pre-existing HI titer.41 Pre-vaccination history was not assessed in this phase I study. Thus, correlations between immune response and previous vaccination or pre-existing HI titer should be investigated in further clinical trials. Over 50% (91 volunteers) of the total volunteers in this study were excluded because of high pre-existing HI titers.
Adverse reactions from inactivated split influenza vaccines may differ according to the chemical agents used for inactivation and splitting of viruses, type of detergent, degree of splitting, neuraminidase concentration, endotoxins, and contaminants. 44454647 In a meta-analysis of about 30 studies on inactivated split influenza vaccines, Beyer, et al.48 reported that severe adverse reactions were very rare. In the present study, a total of 51 adverse events that could have been causally related to the study vaccine occurred in 29 subjects (65.91%). There were no severe adverse events above grade 3, and all the adverse events eventually resolved. Two subjects had abnormal vital signs and laboratory results; however, all the abnormal findings eventually returned to normal without any interventions.
In a previous clinical trial in Korean adults of an inactivated split influenza vaccine manufactured in 2008, Song, et al.49 reported the occurrence rates of solicited local adverse events and solicited systemic adverse events as 61.0% and 39.8%, respectively. 34 The present study showed a frequency of solicited local adverse events (47.7%) lower than that observed in the previous study and a frequency of solicited systemic adverse events (36.4%) similar to that reported in the previous study. Injection site pain was most common among the solicited local adverse events, in accordance with previous reports. Fatigue was most common among the solicited systemic adverse events, although headache and myalgia have been reported as the most common adverse events in previous reports.4950
In conclusion, TIV was found to be tolerable and sufficiently immunogenic in healthy subjects. However, its safety and immunogenicity should be confirmed in large-scale clinical studies.
Figures and Tables
Table 1
The Results of Immunogenicity
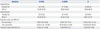
Table 2
Solicited and Unsolicited Adverse Events on Days 0–14

Table 3
Follow Up Results of Vital Signs and Laboratory Findings on Day 1, Day 14, and Day 28 (Final Visit)

ACKNOWLEDGEMENTS
This phase I trial was sponsored by the IL-YANG Pharmaceutical Company in South Korea. We thank the volunteers who participated in this study and the staff of the Vaccine Bio Research Institute of the Catholic University of Korea who supported this study.
References
1. World Health Organization. Influenza. accessed on 2016 Mar 1. Available at: http://www.who.int/mediacentre/factsheets/fs211/en.
2. Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007; 56:1–54.
3. Sullivan KM. Health impact of influenza in the United States. Pharmacoeconomics. 1996; 9:Suppl 3. 26–33.

4. Centers for Disease Control and Prevention (CDC). Key facts about influenza (flu) & flu vaccine. accessed on 2016 Jan 10. Available at: http://www.cdc.gov/flu/keyfacts.htm.
5. de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005; 353:2667–2672.

6. Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, Nguyen KH, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005; 437:1108.
7. Influenza vaccines. Wkly Epidemiol Rec. 2002; 77:230–239.
8. Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003; 21:1769–1775.

9. Pyhälä R, Alanko S, Forsten T, Haapa K, Kinnunen L, Jääskivi M, et al. Early kinetics of antibody response to inactivated influenza vaccine. Clin Diagn Virol. 1994; 1:271–278.

10. Gulati U, Kumari K, Wu W, Keitel WA, Air GM. Amount and avidity of serum antibodies against native glycoproteins and denatured virus after repeated influenza whole-virus vaccination. Vaccine. 2005; 23:1414–1425.

11. Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004; 103:133–138.

12. Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979; 35:69–75.

13. Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. accessed on 2016 Apr 30. Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074775.htm.
14. Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. accessed on 2016 Apr 30. Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074794.htm.
15. Feng SZ, Jiao PR, Qi WB, Fan HY, Liao M. Development and strategies of cell-culture technology for influenza vaccine. Appl Microbiol Biotechnol. 2011; 89:893–902.

16. Minor PD, Engelhardt OG, Wood JM, Robertson JS, Blayer S, Colegate T, et al. Current challenges in implementing cell-derived influenza vaccines: implications for production and regulation, July 2007, NIBSC, Potters Bar, UK. Vaccine. 2009; 27:2907–2913.

17. Schild GC, Oxford JS, de Jong JC, Webster RG. Evidence for host-cell selection of influenza virus antigenic variants. Nature. 1983; 303:706–709.

18. Oxford JS, Corcoran T, Knott R, Bates J, Bartolomei O, Major D, et al. Serological studies with influenza A(H1N1) viruses cultivated in eggs or in a canine kidney cell line (MDCK). Bull World Health Organ. 1987; 65:181–187.
19. Katz JM, Webster RG. Amino acid sequence identity between the HA1 of influenza A (H3N2) viruses grown in mammalian and primary chick kidney cells. J Gen Virol. 1992; 73(Pt 5):1159–1165.

20. Szymczakiewicz-Multanowska A, Groth N, Bugarini R, Lattanzi M, Casula D, Hilbert A, et al. Safety and immunogenicity of a novel influenza subunit vaccine produced in mammalian cell culture. J Infect Dis. 2009; 200:841–848.

21. Reisinger KS, Block SL, Izu A, Groth N, Holmes SJ. Subunit influenza vaccines produced from cell culture or in embryonated chicken eggs: comparison of safety, reactogenicity, and immunogenicity. J Infect Dis. 2009; 200:849–857.

22. Groth N, Montomoli E, Gentile C, Manini I, Bugarini R, Podda A. Safety, tolerability and immunogenicity of a mammalian cell-culture-derived influenza vaccine: a sequential Phase I and Phase II clinical trial. Vaccine. 2009; 27:786–791.

23. Nakamura K, Homma M. Protein synthesis in Vero cells abortively infected with influenza B virus. J Gen Virol. 1981; 56(Pt 1):199–202.

24. Lau SC, Scholtissek C. Abortive infection of Vero cells by an influenza A virus (FPV). Virology. 1995; 212:225–231.

25. Robertson JS, Cook P, Attwell AM, Williams SP. Replicative advantage in tissue culture of egg-adapted influenza virus over tissue-culture derived virus: implications for vaccine manufacture. Vaccine. 1995; 13:1583–1588.

26. Roedig JV, Rapp E, Höper D, Genzel Y, Reichl U. Impact of host cell line adaptation on quasispecies composition and glycosylation of influenza A virus hemagglutinin. PLoS One. 2011; 6:e27989.

27. Lin YP, Wharton SA, Martín J, Skehel JJ, Wiley DC, Steinhauer DA. Adaptation of egg-grown and transfectant influenza viruses for growth in mammalian cells: selection of hemagglutinin mutants with elevated pH of membrane fusion. Virology. 1997; 233:402–410.

28. Hess RD, Weber F, Watson K, Schmitt S. Regulatory, biosafety and safety challenges for novel cells as substrates for human vaccines. Vaccine. 2012; 30:2715–2727.

29. Perdue ML, Arnold F, Li S, Donabedian A, Cioce V, Warf T, et al. The future of cell culture-based influenza vaccine production. Expert Rev Vaccines. 2011; 10:1183–1194.

30. Schultz-Cherry S, Jones JC. Influenza vaccines: the good, the bad, and the eggs. Adv Virus Res. 2010; 77:63–84.
31. Baxter R, Patriarca PA, Ensor K, Izikson R, Goldenthal KL, Cox MM. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok® trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50-64 years of age. Vaccine. 2011; 29:2272–2278.

32. López-Macías C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, Talavera J, Arteaga-Ruiz O, et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine. 2011; 29:7826–7834.

33. Rappuoli R, Giudice GD. Influenza vaccines for the future. 2nd ed. Basel: Springer;2011.
34. Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005; 23:1029–1036.

35. Hegde NR. Cell culture-based influenza vaccines: a necessary and indispensable investment for the future. Hum Vaccin Immunother. 2015; 11:1223–1234.

36. Bandell AR, Simões EA. Live attenuated influenza vaccine tetravalent: a clinical review. Expert Rev Vaccines. 2015; 14:963–973.

37. Valero-Pacheco N, Pérez-Toledo M, Villasís-Keever MÁ, Núñez-Valencia A, Boscó-Gárate I, Lozano-Dubernard B, et al. Antibody persistence in adults two years after vaccination with an H1N1 2009 pandemic influenza virus-like particle vaccine. PLoS One. 2016; 11:e0150146.

38. Dormitzer PR, Tsai TF, Del Giudice G. New technologies for influenza vaccines. Hum Vaccin Immunother. 2012; 8:45–58.

39. Hirota Y, Kaji M, Ide S, Goto S, Oka T. The hemagglutination inhibition antibody responses to an inactivated influenza vaccine among healthy adults: with special reference to the prevaccination antibody and its interaction with age. Vaccine. 1996; 14:1597–1602.

40. Beyer WE, Palache AM, Sprenger MJ, Hendriksen E, Tukker JJ, Darioli R, et al. Effects of repeated annual influenza vaccination on vaccine sero-response in young and elderly adults. Vaccine. 1996; 14:1331–1339.

41. Nabeshima S, Kashiwagi K, Murata M, Kanamoto Y, Furusyo N, Hayashi J. Antibody response to influenza vaccine in adults vaccinated with identical vaccine strains in consecutive years. J Med Virol. 2007; 79:320–325.

42. Gross PA, Sperber SJ, Donabedian A, Dran S, Morchel G, Cataruozolo P, et al. Paradoxical response to a novel influenza virus vaccine strain: the effect of prior immunization. Vaccine. 1999; 17:2284–2289.

43. Iorio AM, Camilloni B, Basileo M, Neri M, Lepri E, Spighi M. Effects of repeated annual influenza vaccination on antibody responses against unchanged vaccine antigens in elderly frail institutionalized volunteers. Gerontology. 2007; 53:411–418.

44. Kelly HA, Skowronski DM, De Serres G, Effler PV. Adverse events associated with 2010 CSL and other inactivated influenza vaccines. Med J Aust. 2011; 195:318–320.

45. Skowronski DM, Strauss B, De Serres G, MacDonald D, Marion SA, Naus M, et al. Oculo-respiratory syndrome: a new influenza vaccine-associated adverse event? Clin Infect Dis. 2003; 36:705–713.

46. Nicholson KG, Webster RG, Hay AJ. Textbook of influenza. Oxford: Blackwell Science;1998.
47. Therapeutic Goods Administration. Seasonal flu vaccine: overview of vaccine regulation and safety monitoring and investigation into adverse events following 2010 seasonal influenza vaccination in young children. accessed on 2016 Jan 10. Available at: https://www.tga.gov.au/node/581.
48. Beyer WE, Nauta JJ, Palache AM, Giezeman KM, Osterhaus AD. Immunogenicity and safety of inactivated influenza vaccines in primed populations: a systematic literature review and meta-analysis. Vaccine. 2011; 29:5785–5792.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download