Abstract
The causes of cytopenia in patients with severe fever with thrombocytopenia syndrome (SFTS) are not fully understood until now. We reviewed the bone marrow (BM) findings of patients with SFTS to unravel the cause of the cytopenia. Three Korean SFTS were enrolled in this study. Thrombocytopenia, neutropenia, and anemia were detected in all three patients. Severe hypocellular marrow (overall cellularity <5%) and a decreased number of megakaryocytes were noted in one patient, and hypo-/normocellular marrow and an increased number of hemophagocytic histiocytes were observed in two patients. Megakaryocytes were relatively preserved in two patients. Although a limited number of cases are available, our observations suggest that both BM suppression and peripheral destruction or sequestration are causes of cytopenia of patients with SFTS. To the best of our knowledge, this is the first well documented pathologic evaluation of Korean SFTS.
Severe fever with thrombocytopenia syndrome (SFTS) is a fatal infectious disease caused by the SFTS virus (SFTSV), which is a novel Phlebovirus in the family Bunyavirida.1 The major clinical features of patients with SFTS are high fever, thrombocytopenia, leukopenia, and gastrointestinal symptoms. Elevated serum levels of alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, lactate dehydrogenase, creatine kinase, and ferritin are also common laboratory findings in patients with SFTS. However, the pathological mechanism of thrombocytopenia and leukopenia in patients with SFTS is not fully understood until now; it is unclear whether production failure or peripheral destruction/sequestration is the main mechanism of cytopenia in these patients.234
In the present study, therefore, we investigated the bone marrow (BM) findings of patients with SFTS to understand the pathogenesis of SFTS.
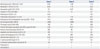
Abdominal pain developed in a 73-year-old man. He was transfused with packed red blood cells due to low hemoglobin (Hb) (7.6 g/dL) at a local hospital 10 days later. Fever, neutropenia, and elevated liver enzymes were observed. He was referred to our hospital for further evaluation and treatment. SFTSV was confirmed by reverse-transcription polymerase chain reaction (RT-PCR) analysis5 at Division of Arboviruses, National Institute of Health, Korea Centers for Disease Control and Prevention. The laboratory and clinical findings are summarized in Table 1 and 2. A BM biopsy was performed. Hemophagocytic histiocytes were observed in an aspirate (Fig. 1A), and hypocellular marrow was noted in the BM section (Fig. 1B). However, megakaryocytes were relatively preserved in the section (Fig. 1C). He was treated with antibiotics and plasmapheresis. However, he died 3 weeks after the initial symptoms (3 days after BM biopsy) due to metabolic acidosis and multi-organ failure.
Fever and enlargement of the left inguinal lymph node developed in a 53-year-old man, and he was treated with antibiotics at a local hospital. However, pancytopenia was detected (Hb, 12.9 g/dL; white blood cell, 3.77×109/L; platelet, 24.0×109/L) at local hospital, and he was referred to our hospital for further evaluation and treatment. SFTSV was confirmed by RT-PCR analysis 5 at Division of Arboviruses, National Institute of Health, Korea Centers for Disease Control and Prevention. The laboratory and clinical findings at our hospital are summarized in Table 1 and 2. Severe hypocellular marrow was noted in an aspirate and section (Fig. 1D and E). Megakaryocytes were rarely found. He was treated with antibiotics; however, he died 10 days after admission due to multi-organ failure.
An 86-year-old woman was admitted to our hospital for a 3 day fever. She had been with antibiotics at local hospital; however, pancytopenia was detected, and she was referred to our hospital for further evaluation and treatment. SFTSV was confirmed by RT-PCR analysis5 at Division of Arboviruses, National Institute of Health, Korea Centers for Disease Control and Prevention. The laboratory and clinical findings at our hospital are summarized in Table 1 and 2. Hemophagocytic histiocytes were observed in an aspirate (Fig. 1F). The megakaryocytes were normally observed in an aspirate (Fig. 1G). Normocellular marrow with focal hypocellular area was noted in the BM section (Fig. 1H). The patient was successfully treated with antibiotics and ribavirin.
Thrombocytopenia and leukopenia are prominent features in patients with SFTS. Viral replication in a mouse model mainly occurs in splenic macrophages.4 However, SFTSV is not found in mice BM,4 but the numbers of megakaryocytes increase in the spleen and BM of mice.4 In vitro cell assays show that SFTSV adheres to mouse platelets and facilitates phagocytosis of platelets by primary macrophages, suggesting that the cause of the thrombocytopenia is destruction by splenic macrophages.4 QuanTai, et al.2 compared the BM findings of five Chinese patients with SFTS with those of patients with aplastic anemia and normal healthy volunteers, and found no significant differences in cell morphology, cellularity, or numbers of megakaryocytes between them. They concluded that peripheral blood thrombocytopenia and leukopenia in patients with SFTS result from increased peripheral organ damage or circulating anti-platelet antibodies.2
However, hypocellular marrow with an increased number of hemophagocytic histiocytes is observed in Japanese patients with SFTS,3 whereas megakaryocytes are relatively preserved in BM.3 Consistent with these findings, our Korean patients also showed moderate to severe hypocellular marrow with an increased number of hemophagocytic histiocytes and/or relatively preserved megakaryocytes. The reason for the different BM findings between Chinese and Japanese or Korean patients with SFTS is unclear. The Chinese patients with SFTS were relatively young age (30–50 years) and all of them recovered successfully.2 However, our Korean patients with SFTS (53–86 years) and the Japanese patients with SFTS were older (>50 years), and two of our Korean patients died. Therefore, age and clinical status/severity may be the cause of the different BM findings.67
Deng, et al.8 also observed that two patients expired of SFTS presented with empty marrow. These two8 cases and our cases (case 1 and case 2) suggest that BM hypocellularity is associated with severity of SFTS. However, further studies are needed.
Considering the results of animal experiments4 and those of pathological examinations of patients with SFTS,23 hemophagocytosis appears to be common in patients with SFTS, and the laboratory findings of most patients with SFTS are compatible with hemophagocytic lymphohistocytosis (fever, cytopenia, high ferritin level, etc.).79 Moreover, one study revealed that increased cytokine levels are correlated with viral load/clinical parameters in patients with SFTS.10 Since dysregulation of the immune system with hypercytokinemia is an underlying mechanism of hemophagocytic lymphohistocytosis,11 SFTSV may produce hemophagocytic lymphohistocytosis.9 Therefore, cytopenia in patients with SFTS may result from both peripheral destruction/sequestration and BM suppression.
Based on the "cytokine storm" and "immune-mediated platelet consumption in the spleen" concepts, some authors have reported cases treated with plasmapheresis to reduce cytokine levels as well as other pathological immune-mediating agents.12 They reported two patients treated with ribavirin and plasmapheresis and they recovered from SFTS.12 However, plasmapheresis does not have demonstrated therapeutic efficacy until now. Our Patient 1 died after a plasmapheresis treatment. Therefore, further studies are needed to define the exact pathogenesis and the therapeutic implications.
In conclusion, our results together with other studies indicate that BM suppression and hemophagocytic histiocytes are common findings in patients with SFTS. Although a limited number of cases were available, our observations may help understand the pathogenic mechanism of SFTSV and aid in future therapeutic applications.
Figures and Tables
Fig. 1
Findings of bone marrow (BM) aspirate and section of case 1 (A, B, and C), 2 (D and E), and 3 (F, G, and H). (A) The hemophagocytic histiocytes were increased in the aspirate [Wright-Giemsa (W-G), ×400]. (B) Hypocellular area was noted in the BM section [hematoxylin and eosin (H&E), ×100]. (C) The number of dysplastic megakaryocytes increased slightly in the cellular area (CD61 immunohistochemistry, ×400). (D) Hypocellular particles (W-G, ×40) are noted. (E) Severe hypocellular marrow is noted (H&E, ×40). (F) The hemophagocytic histiocytes are increased in the aspirate (W-G, ×400). (G) Megakaryocytes are normally observed in the aspirate (W-G, ×200). (H) Normocellular marrow for age (86 years) with a focally hypocellular area is noted (H&E, ×100).
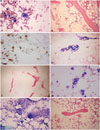
Table 1
Basic Characteristics of the Patients with Severe Fever with Thrombocytopenic Syndrome (SFTS)
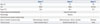
Table 2
Laboratory Findings of the Patients with Severe Fever with Thrombocytopenia Syndrome
References
1. Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011; 364:1523–1532.

2. QuanTai X, FengZhe C, XiuGuang S, DongGe C. A study of cytological changes in the bone marrow of patients with severe fever with thrombocytopenia syndrome. PLoS One. 2013; 8:e83020.

3. Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis. 2014; 209:816–827.

4. Jin C, Liang M, Ning J, Gu W, Jiang H, Wu W, et al. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc Natl Acad Sci U S A. 2012; 109:10053–10058.

5. Kim WY, Choi W, Park SW, Wang EB, Lee WJ, Jee Y, et al. Nosocomial transmission of severe fever with thrombocytopenia syndrome in Korea. Clin Infect Dis. 2015; 60:1681–1683.

6. Ding S, Niu G, Xu X, Li J, Zhang X, Yin H, et al. Age is a critical risk factor for severe fever with thrombocytopenia syndrome. PLoS One. 2014; 9:e111736.

7. Gai ZT, Zhang Y, Liang MF, Jin C, Zhang S, Zhu CB, et al. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis. 2012; 206:1095–1102.

8. Deng B, Zhou B, Zhang S, Zhu Y, Han L, Geng Y, et al. Clinical features and factors associated with severity and fatality among patients with severe fever with thrombocytopenia syndrome Bunyavirus infection in Northeast China. PLoS One. 2013; 8:e80802.

9. Jordan MB, Filipovich AH. Hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis: a journey of a thousand miles begins with a single (big) step. Bone Marrow Transplant. 2008; 42:433–437.

10. Sun Y, Jin C, Zhan F, Wang X, Liang M, Zhang Q, et al. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J Infect Dis. 2012; 206:1085–1094.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download