Abstract
Purpose
Materials and Methods
Results
Figures and Tables
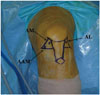 | Fig. 1Anterolateral (AL), anteromedial (AM), and accessory anteromedial (AAM) portals were established. The AM portal was used as a viewing portal for inspecting femoral ACL insertion sites. Femoral AM and PL bone tunnels were made through the AAM portal. PL, posterolateral; ACL, anterior cruciate ligament. |
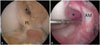 | Fig. 2Arthroscopic view from anteromedial portal. (A) The PL bundle was elongated, but still bridged tibia and femur (PL), although the AM bundle was completely torn (*). Selective AM bundle reconstruction was performed in this case. (B) A completely torn PL bundle was noted (*) by probing an AM bundle that was moderately attenuated (AM). Selective PL bundle reconstruction was conducted in this case. PL, posterolateral; AM, anteromedial. |
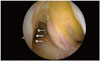 | Fig. 3Arthroscopic view from anterolateral portal. Both AM and PL bundles were absent (arrows). DB ACL reconstruction was conducted in this case. AM, anteromedial; PL, posterolateral; DB, double bundle; ACL, anterior cruciate ligament. |
 | Fig. 4Arthroscopic view from anteromedial portal. (A) The AM graft (AM) was passed after PL graft (PL) fixation during DB ACL reconstruction. (B) Reconstructed AM bundle (AM) and (C) reconstructed PL bundle (PL). AM, anteromedial; PL, posterolateral; DB, double bundle; ACL, anterior cruciate ligament. |
Table 1
Patient Demographics (n=98)

Table 2
Graft Diameter and Tunnel Length (n=98)

Table 3
Pre- and Post-Operative Anterior Laxity Measurements (n=98)

STS, side to side; DB, double bundle; AM, anteromedial; PL, posterolateral; NS, not significant; HSD, honestly significant difference.
Postoperative data significantly improved in all three study groups from preoperative data (all p<0.001). Regarding intergroup differences, group A (DB group) showed significantly greater preoperative laxity measured by KT-2000 and stress X-ray compared to groups B and C (AM and PL bundle group), respectively.
*p value was derived from ANOVA, †‡§p values were derived from Tukey's HSD post-hoc test.
Table 4
Pre- and Post-Operative Manual Instability Assessment (n=98)

DB, double bundle; AM, anteromedial; PL, posterolateral; NS, not significant; HSD, honestly significant difference.
Group A (DB group) exhibited significantly greater preoperative rotational instability checked by pivot shift test, compared to groups B and C (AM and PL bundle group), respectively (all p<0.001).
*p value was derived from ANOVA, †‡§p values were derived from Tukey's HSD post-hoc test, †pDB vs. AM=NS (between group A and group B), ‡pDB vs. PL=0.008 (between group A and group C), §pAM vs. PL=NS (between group B and group C), †pDB vs. AM<0.001 (between group A and group B), ‡pDB vs. PL<0.001 (between group A and group C), §pAM vs. PL=NS (between group B and group C).
Table 5
Functional Score Assessment (n=98)

DB, double bundle; AM, anteromedial; PL, posterolateral; NS, not significant.
Pre- and postoperative IKDC and Lysholm scores. Significant postoperative improvements were observed in the three study groups (all p<0.001), although no significant intergroup difference was found postoperatively.
*pDB vs. AM=NS (between group A and group B), †pDB vs. PL=0.045 (between group A and group C), ‡pAM vs. PL=NS (between group B and group C).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download