Abstract
Purpose
We report our initial experience with transurethral injection of autologous adipose-derived regenerative cells (ADRCs) for the treatment of urinary incontinence after radical prostatectomy.
Materials and Methods
After providing written informed consent, six men with persistent urinary incontinence after radical prostatectomy were enrolled in the study. Under general anesthesia, about 50 mL of adipose tissue was obtained from the patients by liposuction. ADRCs were obtained by separation with centrifugation using the Celution cell-processing device. A mixture of ADRCs and adipose tissue were transurethrally injected into the submucosal space of the membranous urethra. Functional and anatomical improvement was assessed using a 24-h pad test, validated patient questionnaire, urethral pressure profile, and magnetic resonance imaging (MRI) during 12-week follow-up.
Results
Urine leakage volume was improved with time in all patients in the 24-h pad test, with the exemption of temporal deterioration at the first 2 weeks post-injection in 2 patients. Subjective symptoms and quality of life assessed on the basis of questionnaire results showed similar improvement. The mean maximum urethral closing pressure increased from 44.0 to 63.5 cm H2O at 12 weeks after injection. MRI showed an increase in functional urethral length (from 6.1 to 8.3 mm) between the lower rim of the pubic bone and the bladder neck. Adverse events, such as pelvic pain, inflammation, or de novo urgency, were not observed in any case during follow-up.
Stress urinary incontinence (SUI) is common among men, particularly following radical prostatectomy. Depending on the severity of postoperative SUI and on the patient's preference, various treatment options are available. They include pharmacological treatment, surgical treatment (e.g., injection therapy with bulking agents), application of sling systems, and cell therapy.1 All of these methods involve some limitations, and therefore new, innovative, and experimental approaches have been proposed as alternative treatment options. Stem cells are a self-renewing group of cells derived from tissue that can differentiate into various other cells. Ideally, stem cell therapy to treat SUI would enable the functional periurethral tissue regeneration to provide proper mucosal coaptation and recover resting urethral closure pressure.2 Multiple animal model studies have reported on the effect of stem cells on regeneration of tissue in the urethra, and more recently, some human trials have also been conducted.345 Recent studies have shown that adipose-derived stem cells (ASCs) can differentiate into various type of cells, such as myoblasts, fibroblasts, endothelial cells, smooth muscle cells, or neurogenic cells.6 With regard to treatment of SUI, ASCs are of special concern for promoting revascularization and neuronal and mesodermal regeneration. In fact, neural-differentiated ASCs present glial characteristics and facilitate nerve regeneration, as observed in transplanted rat models.7 Furthermore, periurethral injection of ASCs improved urethral resistance and showed in vivo differentiation into smooth muscle cells.89 In addition, cultured ASCs secrete various angiogenesis-related cytokines, including vascular endothelial growth factor and hepatocyte growth factor.10 Gotoh, et al.11 reported their experience with periurethral injection of adipose-derived regenerative cells (ADRCs) in 11 patients with SUI after radical prostatectomy. In their study, they used the Celution system (Cytori Therapeutics, San Diego, CA, USA), which is a commercially available equipment that allows rapid isolation of therapeutic doses of autologous ADRCs from human adipose tissue following liposuction, thus eliminating the need for culture.12
Using this machine, we developed a new cell therapy for SUI due to urethral sphincter deficiency. The cell therapy included periurethral injection of autologous ADRCs. In this study, we investigated whether ADRC injection therapy for patients with persistent SUI after radical prostatectomy can be duplicated to show similar efficacy and safety as that reported by Gotoh, et al.11 in patients with persistent SUI after radical prostatectomy.
This study was approved by the Ethics Committee of Kyungpook National University School of Medicine, and written informed consent was obtained from all patients.
In the present study, we enrolled six SUI patients after radical prostatectomy. The inclusion criteria were as follows: persistent urinary incontinence for >1 year after surgery, need for pad use, no evidence of metastasis or recurrence of prostate cancer, and need for further therapy other than pharmacologic treatment.
Under general anesthesia, liposuction of 200 mL of adipose tissue was applied along the abdominal wall after making a single periumbilical incision. ADRCs were isolated from the suctioned adipose tissue by the Celution system. The Celution cell-processing device extracts and concentrates the mononuclear fraction of adipose tissue automatically and aseptically, and removes matrix fragments and unwarranted or deleterious cells. It required approximately 1 h to process 250 mL of liposuction tissue. The concentrated cell output was counted using a NucleoCounter (Chemometec, Allerød, Denmark). Finally, we obtained a 5-mL solution containing concentrated ADRCs.12
For periurethral injection of ADRCs, two distinct formulations were prepared: an isolated, 1-mL ADRC fraction was prepared for direct injection, and another 4-mL fraction was mixed with 16 mL of intact autologous adipose cells, producing 20-mL combined solution. A 24 Fr rigid endoscope was used to inject the processed ADRC solution. The endoscope was inserted into the urethra. Under endoscopic vision, an injection needle was inserted through the endoscope into the urethral mucosa at the external sphincter. After successful puncture, the ADRC solution was injected. First, the 1-mL isolated ADRCs solution was injected into the rhabdosphincter at a 5 mm depth in the 5 and 7 o'clock directions. Subsequently, the 20-mL combined solution was injected into the submucosal space in the 4, 6, and 8 o'clock directions to promote a bulking effect along the urethral mucosa and to facilitate complete coaptation. After the injection of all solutions, an 8 Fr Foley catheter was placed. The next day, it was removed to facilitate micturition.
To quantify treatment success rates, patients were evaluated before and after therapy in accordance with four criteria. The degree of incontinence was evaluated using a 24-h pad test, and the daily total leakage amount was measured. During each evaluation period, the 24-h pad test was repeated three times. This parameter was evaluated at baseline and at 2, 4, 8, and 12 weeks after procedure. The subjective symptoms and quality of life were assessed by the International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF), a validated disease-specific questionnaire. In the ICIQ-SF, the therapeutic effects in aspects of frequency of incontinence (0–5 point scores), leakage amount (0–6 point scores), and impact on daily life (0–10 point scores) were examined, and the total score (0 to 21 points score) was calculated. A high score indicated an unfavorable condition. This parameter was assessed at baseline and at 4 and 12 weeks after the procedure. Urodynamic tests, including urethral pressure profiles and pressure flow studies, were performed to determine whether lower urinary tract obstruction occurred after therapy and to prove the effect of therapy on urethral closure pressure. Maximum urethral closing pressure (MUCP) and functional profile length (FPL) were measured at baseline and at 4 and 12 weeks after the procedure. Urethral sphincter thickness was monitored with magnetic resonance imaging (MRI) by measuring the length between the lower rim of the pubic bone and bladder neck. These imaging studies were carried out at baseline and at 4 and 12 weeks after treatment.
Transurethral injection of ADRCs was performed in all 6 patients without perioperative complications. After removal of the Foley catheter, all patients could micturate without significant residual urine. No patient had abnormal voiding symptoms. The urethral lumen was completely closed after the periurethral injection at the external urethral sphincter (Fig. 1). In 2 patients, urinary incontinence deteriorated within 2 weeks after injection. However, urinary incontinence progressively improved thereafter in these 2 patients, and continuously improved in the other 4 patients for 12 weeks. By 12 weeks after treatment, urinary incontinence in aspects of leakage volume, measured by a 24-h pad test, had improved (Table 1). On the basis of the ICIQ-SF questionnaire, subjective symptoms and quality of life reflected similar improvement. Urethral sphincter function improved in all cases. The mean MUCP increased from 44.0 to 63.5 cm H2O (Table 1). MRI showed an increase in the functional urethral length (from 6.1 to 8.3 mm) between the lower rim of the pubic bone and bladder neck (Table 1, Fig. 2). Significant side effects of inflammation, pelvic pain, or de novo urgency were not observed after the procedure in any patient during the postoperative follow-up period.
Embryonic stem cells are multipotent cells derived from the inner cell cluster of blastocysts. They hold the potential to differentiate into cells from any of the three embryonic germ layers. To avoid ethical and political problems that could limit the use of these cells, research into alternative treatments for SUI has focused on using autologous adult-derived stem cells. Unfortunately, adult stem cells are not immortal and have more limited potential of differentiation. Investigations into using stem cells for the treatment of SUI have focused on mesenchymal-derived stem cells. These cells can be isolated from many different sources, such as bone marrow, muscle, adipose tissue, amniotic fluid, liver tissue, dental pulp, placenta, and umbilical cord.2
Very few studies using autologous adult-derived stem cells for the treatment of SUI in humans have been reported in the literature. Even fewer papers have been published concerning male SUI. The first experience was reported by Strasser, et al.13 in 2007 after treating a group of men and women affected by SUI with ultrasonography-guided injection of myoblasts and fibroblasts into the rhabdosphincter and submucosa, respectively. After a follow-up of 12 months, the authors recorded a significant improvement in incontinence and quality of life scores, thickness of the urethra and rhabdosphincter, and the contractility of the rhabdosphincter. These postoperative changes were explained by the formation of new muscle tissue in the rhabdosphincter.1314 This muscle tissue formation has been related to the greater difficulty of injecting MDSCs in men than in women due to the anatomy of the male urethra and postoperative scarring. Mitterberger, et al.15 in 2008 reported their experience using the same technique with a longer follow-up period in a study of 63 male patients with SUI after radical prostatectomy. Significant postoperative improvements in incontinence and quality of life scores, as well as thickness and contractility of the rhabdosphincter with no severe side effects, were recorded at 1 year of follow-up. In 2012, however, Gerullis, et al.16 reported that only 120 of 222 male patients with SUI and sphincter damage after urologic procedures, who were treated with transurethral injection of autologous muscle-derived cells, responded to therapy. Among therapy-responsive men, 26 (12%) were continent, and 94 (42%) showed improvement. For 102 (46%) patients, however, the therapy was ineffective.
A variety of conditions in humans has been successfully treated with ADRCs.171819 In an introductory investigation, Lin, et al.12 reported the characteristics of Celution-isolated ADRCs. Using flow cytometry and colony-forming unit fibroblast assays, the authors showed that cells isolated using the Celution system were composed of a heterogeneous cell population, including ASCs, mature and progenitor endothelial cells, CD45+ hematopoietic cells, vascular smooth muscle cells, resident tissue macrophage/monocytes, preadipocytes, and pericytes, containing ASCs in 0.6–1.6% of all components. Furthermore, to explore the safety and feasibility of ADRC transplantation in myocardial infarction patients, a first-in-man randomized controlled trial is currently in progress in the Netherlands.20
In 2013, Gotoh, et al.11 reported their experience with the periurethral injection of ADRCs in 11 patients with SUI after radical prostatectomy. They reported a progressive improvement in sphincter function, shown by an increase in the MUCP and FPL, as well as decreased leakage volume, shown by a 24-h pad test with no significant adverse events. The authors also explained the advantage of using adipose tissue, which contains multipotent stem cells, as well as progenitor cells and key mature cells. The Celution system allows for rapid and adequate collection of ADRCs from each patient. Unlike other cell therapy procedures, the treatment is completely autologous and requires no cell culture; furthermore, it is carried out in a single surgical procedure.11 Before this study, most information was from several animal studies using rats to confirm the effect of periurethral injection of ASCs on urethral resistance and sequential changes.21
In our study, two patients initially experienced greater urinary incontinence after periurethral injection. Although urinary incontinence increased in the first 2 weeks postoperatively, the 24-h pad test results progressively improved at 4, 8, and 12 weeks of follow-up. This might have resulted from inexperience in administering the injection, potentially leading to transient sphincter injury. In the other four cases, the outcomes of the 24-hr pad test continuously improved. The MCUP, functional urethral length, and ICIQ-SF continuously improved in all cases after surgery (Fig. 3). Gotoh, et al.11 reported that 30% of the injected adipose tissue fraction, which was processed to isolate ADRCs, was composed of lactated Ringer's solution. Absorption of the solution could also be responsible for the temporary deterioration in the condition during the initial weeks. The authors further explained that while the perennial bulking effect suggests the survival and growth of the injected adipose tissue, the positive effects on impaired sphincter function could also originate in the presence and paracrine effect of the injected ASCs within the ADRCs. ASCs can differentiate into mature adipose tissue and possibly into contractile cells, and cultured ASCs are known to secrete quite a lot of angiogenesis-related cytokines.1021
This study showed that transurethral injection of autologous ASCs can be a safe and feasible treatment for SUI after radical prostatectomy. This new treatment modality represents a minimally invasive and highly effective therapeutic approach, although larger studies and those that assess long-term outcomes are needed to confirm the efficacy of this new treatment modality.
Figures and Tables
Fig. 1
Before injection, the external urethral sphincter was open. Solution and adipose tissue was injected into the rhabdosphincter and submucosal space at the 5 and 7 o'clock positions and at the 4, 6, and 8 o'clock positions to facilitate complete coaptation of the urethral mucosa by the bulking effect.
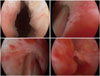
Fig. 2
In case of patient E, MRI showed an increase in the functional urethral length (from 5.07 to 8.59 mm) between the lower rim of the pubic bone and bladder neck 4 weeks after injection (A: baseline, B: 4 wks after injection).
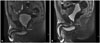
Fig. 3
Changes in 24-h pad test, MUCP, MRI, ICIQ-SF. MUCP, maximum urethral closing pressure; MRI, magnetic resonance imaging; ICIQ-SF, International Consultation on Incontinence Questionnaire-Short Form.

Table 1
Clinical Outcomes

ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (grant number) (2014R1A1A3049460); (NRF-2014M3A9D3033887); funded by the Ministry of Education (2015R1D1A3A03020378); and supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (HI14C1642).
References
1. Bauer RM, Bastian PJ, Gozzi C, Stief CG. Postprostatectomy incontinence: all about diagnosis and management. Eur Urol. 2009; 55:322–333.

2. Clemens JQ, Schuster TG, Konnak JW, McGuire EJ, Faerber GJ. Revision rate after artificial urinary sphincter implantation for incontinence after radical prostatectomy: actuarial analysis. J Urol. 2001; 166:1372–1375.

3. Giberti C, Gallo F, Schenone M, Cortese P, Ninotta G. Stem cell therapy for male urinary incontinence. Urol Int. 2013; 90:249–252.

4. Kinebuchi Y, Aizawa N, Imamura T, Ishizuka O, Igawa Y, Nishizawa O. Autologous bone-marrow-derived mesenchymal stem cell transplantation into injured rat urethral sphincter. Int J Urol. 2010; 17:359–368.

5. Zhao W, Zhang C, Jin C, Zhang Z, Kong D, Xu W, et al. Periurethral injection of autologous adipose-derived stem cells with controlled-release nerve growth factor for the treatment of stress urinary incontinence in a rat model. Eur Urol. 2011; 59:155–163.

6. Roche R, Festy F, Fritel X. Stem cells for stress urinary incontinence: the adipose promise. J Cell Mol Med. 2010; 14:135–142.

7. Zeng X, Jack GS, Zhang R. Treatment of SUI using adipose derived stem cells: restoration of urethral function. J Urol. 2006; 175:291.

8. Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, et al. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials. 2010; 31:5312–5324.

9. Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010; 12:88–95.

10. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004; 109:1292–1298.

11. Gotoh M, Yamamoto T, Kato M, Majima T, Toriyama K, Kamei Y, et al. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose-derived regenerative cells: 1-year outcomes in 11 patients. Int J Urol. 2014; 21:294–300.

12. Lin K, Matsubara Y, Masuda Y, Togashi K, Ohno T, Tamura T, et al. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008; 10:417–426.

13. Strasser H, Marksteiner R, Margreiter E, Mitterberger M, Pinggera GM, Frauscher F, et al. Transurethral ultrasonography-guided injection of adult autologous stem cells versus transurethral endoscopic injection of collagen in treatment of urinary incontinence. World J Urol. 2007; 25:385–392.

14. Strasser H, Marksteiner R, Margreiter E, Pinggera GM, Mitterberger M, Frauscher F, et al. Autologous myoblasts and fibroblasts versus collagen for treatment of stress urinary incontinence in women: a randomised controlled trial. Lancet. 2007; 369:2179–2186.

15. Mitterberger M, Marksteiner R, Margreiter E, Pinggera GM, Frauscher F, Ulmer H, et al. Myoblast and fibroblast therapy for post-prostatectomy urinary incontinence: 1-year followup of 63 patients. J Urol. 2008; 179:226–231.

16. Gerullis H, Eimer C, Georgas E, Homburger M, El-Baz AG, Wishahi M, et al. Muscle-derived cells for treatment of iatrogenic sphincter damage and urinary incontinence in men. ScientificWorldJournal. 2012; 2012:898535.

17. García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005; 48:1416–1423.

18. Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002; 99:8932–8937.

19. Yoshimura K, Matsumoto D, Gonda K. A clinical trial of soft-tissue augmentation by lipoinjection with adipose-derived stromal cells (ASC). In : International Fat Applied Technology Society (IFATS); Third Annual Meeting; 2005. p. 9–10.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download