Abstract
Purpose
Patients with gout are similar to those with bacterial infection in terms of the nature of inflammation. Herein we compared the differences in procalcitonin (PCT) levels between these two inflammatory conditions and evaluated the ability of serum PCT to function as a clinical marker for differential diagnosis between acute gouty attack and bacterial infection.
Materials and Methods
Serum samples were obtained from 67 patients with acute gouty arthritis and 90 age-matched patients with bacterial infection. Serum PCT levels were measured with an enzyme-linked fluorescent assay.
Results
Serum PCT levels in patients with acute gouty arthritis were significantly lower than those in patients with bacterial infection (0.096±0.105 ng/mL vs. 4.94±13.763 ng/mL, p=0.001). However, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels showed no significant differences between the two groups. To assess the ability of PCT to discriminate between acute gouty arthritis and bacterial infection, the areas under the curves (AUCs) of serum PCT, uric acid, and CRP were 0.857 [95% confidence interval (CI), 0.798–0.917, p<0.001], 0.808 (95% CI, 0.738–0.878, p<0.001), and 0.638 (95% CI, 0.544–0.731, p=0.005), respectively. There were no significant differences in ESR and white blood cell counts between these two conditions. With a cut-off value of 0.095 ng/mL, the sums of sensitivity and specificity of PCT were the highest (81.0% and 80.6%, respectively).
Acute gouty attack is an inflammatory response to monosodium urate crystal deposition. The inflammation usually occurs abruptly. It is characterized by redness, tenderness, swelling, and heat over inflamed joints, and systemic fever can be present during this condition. These clinical features, in addition to laboratory findings including leukocytosis and increases in the serum erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels, are similar to those of infectious diseases. Therefore, differential diagnosis between acute gouty attack and bacterial infection by clinicians is occasionally difficult. Hyperuricemia is one of the characteristics that can be used to differentiate between acute gouty attack and bacterial infection. However, in certain cases, serum uric acid levels increase during bacterial infection yet not during an acute gouty attack,1 making the differential diagnosis more difficult.
Procalcitonin (PCT) is a 13-kDa protein produced by C cells of the thyroid gland in the form of a calcitonin precursor.2 PCT production has been reported to occur in other organs, particularly during bacterial infection.34 Several studies reported that PCT could function as an acute-phase reactant during bacterial infection. PCT is regarded as an important biomarker for the early diagnosis of sepsis in critically ill patients.23456 Recently, several studies have reported the role of PCT in differential diagnosis between bacterial infection and disease flares in autoimmune diseases.789
However, no previous studies have reported the role of PCT in acute gouty attack. Wang, et al.10 reported that the levels of PCT in the serum and synovial fluid of patients with septic arthritis were higher than those of patients with rheumatoid arthritis, osteoarthritis, and gouty arthritis. However, in that study, only 11 patients with gouty arthritis were enrolled, and it was not reported whether any of these patients experienced acute gouty attack.10 Therefore, the objective of this study was to assess whether PCT levels increase in patients with acute gouty attack. In addition, we evaluated the ability of PCT to function as a marker for differential diagnosis between acute gouty attack and bacterial infection.
This cross-sectional study included 67 patients with acute gouty attack and 90 age-matched patients who were diagnosed with bacterial infection. Gout was diagnosed in accordance with the revised American College of Rheumatology criteria.11 The patients with acute gouty attack who were included in this study had symptoms for less than 7 days. Consecutive patients with acute gouty attack were enrolled from inpatient and outpatient clinics at Chung-Ang University Hospital in Seoul, Korea between January 1, 2013 and December 31, 2013. PCT data from patients with bacterial infection were collected retrospectively. The enrollment criteria of patients with bacterial infection were as follows: 1) medical records in the same hospital as the gout patients, 2) diagnosis of bacterial infection, 3) no previous history of gout, 4) no concurrent urate-lowering therapy, 5) PCT level measurements, and 6) successful age-matching with the gout patients. The diagnosis of bacterial infection was confirmed via bacterial culture and/or Gram stain or using the diagnostic criteria of each infection type.12131415161718 A subgroup analysis was conducted for patients with serum uric acid levels <6.0 mg/dL. This study was approved by the Institutional Review Board (IRB) of Chung-Ang University Hospital (C2014248 [1445]). Participants provided their written informed consent to participate in this study, and the IRB approved this consent procedure.
Clinical information was recorded at diagnosis. Clinical and laboratory data for acute gouty attack patients were collected at the time of serum sampling, including measurements of PCT, ESR, CRP, complete blood count, uric acid, blood urea nitrate, creatinine, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), in addition to other laboratory tests. A test for PCT was performed prior to the administration of drugs for gouty attack, such as non-steroidal anti-inflammatory drugs or glucocorticoid. For patients with bacterial infection, clinical and laboratory data were retrospectively obtained from the medical records at the day when the serum PCT was measured. A fluorescent enzyme immunoassay (VIDAS BRA HMS PCT, Marcy, France) was used to measure serum PCT levels according to the manufacturer's recommendations. The assays were performed in duplicates, and the detection limits of serum PCT varied between 0.05 ng/mL and 200 ng/mL.
Continuous variables are expressed as mean±standard deviation. The mean differences between continuous variables were evaluated using Student's t-test and the Mann-Whitney U test. The categorical variables were analyzed using a chi-square test. A multivariate logistic analysis using serum PCT and uric acid levels was performed to obtain the respective odds ratios. The ability of PCT, uric acid, and CRP to distinguish between acute gouty attack and bacterial infection was evaluated using a receiver-operating characteristic (ROC) analysis. The sensitivity and specificity of the estimated best cut-off values were calculated from the ROC curve. In all analyses, a p value of <0.05 was considered to be statistically significant. All statistical analyses were conducted using SPSS Statistics version 20.0 (SPSS Corp., Armonk, NY, USA).
The baseline characteristics of the participants are summarized in Table 1. The mean ages of patients with acute gouty attack and bacterial infection were 53.7±17.1 years and 56.7±19.5 years, respectively. There was no significant difference in age between the two groups.
The disease duration in gout patients was 6.0±9.2 years. The mean symptom duration from initiation of acute gouty attack to blood sampling was 3.0±2.9 days. Thirty-seven out of the 67 patients (55%) in the gout group were taking allopurinol. In patients with bacterial infection, the most common type of infection was pneumonia (37 of 90, 41.1%). The numbers of patients with specific infections were as follows: pharyngotonsillitis, 12 (13.3%); sepsis, 11 (12.2%); cholecystitis or cholangitis, ten (11.1%); urinary tract infection, seven (7.8%); cellulitis, six (6.7%); infectious colitis, four (4.4%); and septic arthritis, three (3.3%).
Serum PCT levels were measured in patients with acute gouty attack and bacterial infection. As shown in Table 1, the PCT levels were significantly higher in patients with bacterial infection than those in patients with acute gouty attack (4.941±13.763 ng/mL vs. 0.096±0.105 ng/mL, p=0.001). More patients in the acute gouty attack group presented serum PCT levels below the lower limit of detection (0.05 ng/mL) than those in the bacterial infection group (46 of 67, 68.7% vs. 13 of 90, 14.4%, p<0.001). The serum uric acid levels in the acute gouty attack group were significantly higher than those in the bacterial infection group (7.62±2.03 mg/dL vs. 5.19±2.36 mg/dL, p<0.001). However, there was no significant difference in ESR level, CRP level, and white blood cell (WBC) count between the two groups (Table 1). In the bacterial infection group, serum PCT levels were not significantly different between male and female patients (6.70±18.73 mg/dL vs. 3.33±6.36 mg/dL, p=0.267).
We performed a subgroup analysis of patients with serum uric acid levels <6.0 mg/dL. The number of patients with low serum uric acid levels was 13 of 67 (19.4%) in the acute gouty attack group and 61 of 90 (67.8%) in the bacterial infection group. The mean ESR level, CRP level, WBC count, and neutrophil count showed no significant differences between the two groups (Table 2). However, the mean serum PCT level in patients with bacterial infection was significantly higher than that in the acute gouty attack patient group (4.738±10.455 ng/mL vs. 0.135±0.102 ng/mL, p=0.002).
To determine the ability of PCT to distinguish between acute gouty attack and bacterial infection, we conducted an ROC analysis of PCT and uric acid for all patients. The areas under the curves (AUCs) of serum PCT, uric acid, and CRP were 0.857 [95% confidence interval (CI), 0.798–0.917, p<0.001), 0.808 (95% CI, 0.738–0.878, p<0.001) and 0.638 (95% CI, 0.544–0.731, p=0.005), respectively (Fig. 1). With a cut-off value of 0.095 ng/mL, the sums of sensitivity and specificity of serum PCT were the highest (81.0% and 80.6%, respectively). The best cut-off value of serum uric acid was 5.75 mg/dL, with a sensitivity of 70.2% and specificity of 85.1%. When serum PCT levels <0.095 ng/mL were regarded as a positive result, the positive predictive value (PPV) and negative predictive value (NPV) in the acute gouty attack group were 75.0% (54 of 72) and 85.7% (72 of 85), respectively. The numbers of true positives, false positives, true negatives, and false negatives were 54, 18, 72, and 13, respectively. In patients with serum uric acid levels ≥6.0 mg/dL, the best cut-off value of serum PCT level was 0.105 ng/mL, and its sensitivity and specificity were 87.0% and 88.9%, respectively (AUC=0.898; 95% CI, 0.814–0.982, p<0.001) (Fig. 2). When serum PCT levels <0.095 ng/mL with serum uric acid levels >5.75 mg/dL were regarded as a positive result, the PPV and NPV in the acute gouty attack group were 92.5% (49 of 53) and 82.7% (86 of 104), respectively. The numbers of true positives, false positives, true negatives, and false negatives were 49, 4, 86, and 18, respectively. On multivariate logistic analysis, the odds ratios for gouty attack in which serum PCT levels <0.095 ng/mL and serum uric acid levels >5.75 mg/dL were 13.555 (95% CI, 5.441–33.771, p<0.001) and 9.940 (95% CI, 3.834–25.770, p<0.001), respectively.
In this study, we assessed firstly whether PCT levels increase in patients with acute gouty attack and then investigated the effectiveness of serum PCT in differential diagnosis between acute gouty attack and bacterial infection. Serum PCT levels were significantly higher in patients with bacterial infection than in those with acute gouty attack. In addition, serum PCT effectively differentiated between acute gouty attack and bacterial infection. To the best of our knowledge, our study is the first report that shows the utility of serum PCT levels in the diagnosis of acute gout patients.
A precise diagnosis between acute gouty attack and infectious diseases is very important, as the management methods for these conditions are distinct and particularly glucocorticoid or canakinumab use in patients with bacterial infection can lead to serious adverse events. Occasionally, clinicians may have difficulties distinguishing between acute gouty attack and bacterial infection, as patients with acute gouty attack and those with bacterial infection show many similarities in terms of the nature of inflammation. The symptoms of acute gouty attack, accompanied by various inflammatory signs such as tenderness, swelling, redness, and occasionally systemic fever, frequently bear resemblance to bacterial infectious diseases. Moreover, laboratory findings are similar for gouty attack and bacterial infection, which may include increased serum ESR or CRP levels and leukocytosis, and in our study, there were no differences in ESR and WBC levels (Table 1). Although the p value of the difference in CRP level was 0.054, the AUC value of CRP was less than that of PCT (Fig. 1). This suggests that serum PCT can be a useful serologic marker for differentiating between acute gouty arthritis and bacterial infections.
Hyperuricemia is also an important factor in the pathophysiology and diagnosis of gout.10 However, acute gouty attacks can occur when serum uric acid levels are within normal limits or when urate crystals are not found in the synovial fluid.119 We performed a subgroup analysis for patients with serum uric acid levels <6.0 mg/dL, as maintaining serum urate below 6.0 mg/dL was generally recommended for prophylaxis of recurrent gouty attack.20 In fact, 13 out of the 61 acute gouty patients (21.3%) in this study had serum uric acid levels <6.0 mg/dL. Nonetheless, serum PCT levels were higher in the bacterial infection group than in the gouty group among patients with serum uric acid levels <6.0 mg/dL (Table 2). ESR and CRP levels and WBC count showed no significant difference between the two groups. These findings indicate that the measurement of serum PCT levels can be useful in differential diagnosis between acute gouty attack and bacterial infection.
PCT is a calcitonin precursor composed of 116 amino acids.2 The normal serum PCT levels in healthy individuals are <0.05 ng/mL or are undetectable.2 However, in systemic inflammations, particularly in bacterial infections, the rapid production of PCT can be directly triggered by microbial endotoxins and indirectly by many proinflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 in various tissues, including lung, liver, kidney, and adipose tissue.32122 After PCT is released to the systemic circulation, its serum level can increase up to 5000 times;32324 these increased levels are detected within 2 to 4 hours, and the maximum levels are found within 6 to 24 hours after stimulation.222 The half-life of PCT is 22 to 26 hours.3 These characteristics can be advantageous when considering the use of PCT as an acute phase reactant to monitor infectious diseases.
The cut-off PCT values for discriminating between bacterial infection and other inflammatory conditions were dependent on the disease type and varied between 0.10 ng/mL and 5.79 ng/mL.6252627 In their systematic review, Schuetz, et al.25 reported a cut-off PCT value between 0.1 ng/mL and 0.5 ng/mL in patients with lower respiratory tract infection.27 In another systematic review, the median cut-off PCT value for differentiating between septic patients and those with systemic inflammatory response syndrome of non-infectious origin was 1.1 ng/mL.6 Our study showed a sensitivity of 81.0% and a specificity of 80.6% for differentiating between bacterial infection and acute gouty attack with a cut-off PCT value of 0.095 ng/mL. The cut-off value among the patients with serum uric acid levels ≥6.0 mg/dL was 0.105 ng/mL, with a sensitivity of 87.0% and specificity of 88.9%, which was similar to 0.095 ng/mL, the cut-off value in all patients. Considering that the PPV for acute gouty attack was 92.5% when low PCT pointing and high uric acid levels were combined, we believe that PCT measurements may be useful for clinicians.
The PCT level was 0.095 ng/mL. Although this amount is small, it might be clinically meaningful in patients with acute gouty arthritis to a considerable extent. Liu, et al.28 reported that the serum PCT level was higher in patients with gouty arthritis than in healthy control without infection. In that study, the serum PCT level in gouty arthritis patients was 0.41±1.23 ng/mL, and particularly, the level in gouty arthritis patients without tophi was 0.10±0.08 ng/mL,28 which was similar to our results. These findings can be explained by various proinflammatory cytokines such as IL-6, TNF-α and particularly IL-1β, which also have important roles in the inflammation caused by acute gouty attack.2930 However, it is unclear why the serum PCT levels do not greatly increase in acute gouty attack patients unlike infectious patients. One possible reason is that microbial toxins such as endotoxins, which are not associated with gout, might have a major role in the release of PCT.331 Unlike what is observed during bacterial infections, increased serum PCT levels were not found in viral infections.32 The absence of an increase in PCT levels in viral infections suggests that viral-stimulated synthesis of interferon-α by macrophages can inhibit TNF-α synthesis.332 Similarly, certain cytokines in patients with gout may inhibit the synthesis of PCT. More studies are needed to elucidate why serum PCT levels do not increase during acute gouty attacks.
One of the limitations of the present study was the small sample size. Therefore, future large-scale studies will help estimate the cut-off PCT values to discriminate between bacterial infection and gout. The second limitation was the heterogeneity of the infection sources. However, there are many similarities in clinical and laboratory findings between the patients with acute gouty attack and those with a variety of bacterial infections, and this study suggests the role of serum PCT in distinguishing acute gouty attack from various bacterial infections. Another limitation was the detection levels of serum PCT. The lower limit of detection in this study was 0.05 ng/mL, and 68.7% (46 of 67) of acute gouty attack patients presented this lower limit. If the serum PCT levels could be detected at <0.05 ng/mL, we might have more precise PCT values. A slightly higher level of baseline AST in the infection group than in the gouty attack group could be another limitation. It would be unrelated to a liver problem, as AST is found in many organs including the liver, heart, skeletal muscle, kidney, and red blood cells, unlike ALT, which is more specific to the liver. In our study, however, serum AST level had no correlation with PCT level. Furthermore, different sex ratios between the two groups–due to the predominance of gout in male patients–may be another limitation. However, there were no reports that serum PCT levels are dependent on sex. In our study, serum PCT levels in the bacterial infection group were not significantly different between male and female patients.
In conclusion, serum PCT levels in patients with acute gouty attack were significantly lower than those in patients with bacterial infection. The best cut-off PCT value to discriminate between infection and gout was 0.095 ng/mL, with a sensitivity of 81.0% and a specificity of 80.6%. Serum PCT is expected to be a useful serologic marker for differential diagnosis between acute gout arthritis and bacterial infection.
Figures and Tables
 | Fig. 1Receiver operating characteristic curves for procalcitonin (PCT), uric acid, and C-reactive protein (CRP) levels. PCT showed better distinguishing ability than uric acid or CRP. |
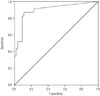 | Fig. 2Receiver operating characteristic curves for procalcitonin (PCT) in patients with serum uric acid levels ≥6.0 mg/dL. PCT showed an ability to distinguish between gouty attack and infection. |
Table 1
Clinical Characteristics and Laboratory Findings of the Study Subjects

Table 2
Clinical Characteristics and Laboratory Findings of Patients with Serum Uric Acid Levels <6.0 mg/dL

References
1. Hershfield MS. Reassessing serum urate targets in the management of refractory gout: can you go too low? Curr Opin Rheumatol. 2009; 21:138–142.

2. Mehanic S, Baljic R. The importance of serum procalcitonin in diagnosis and treatment of serious bacterial infections and sepsis. Mater Sociomed. 2013; 25:277–281.

3. Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med. 2013; 28:285–291.

4. Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006; 34:1996–2003.

5. Nijsten MW, Olinga P, The TH, de Vries EG, Koops HS, Groothuis GM, et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med. 2000; 28:458–461.

6. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013; 13:426–435.

7. Brunkhorst R, Eberhardt OK, Haubitz M, Brunkhorst FM. Procalcitonin for discrimination between activity of systemic autoimmune disease and systemic bacterial infection. Intensive Care Med. 2000; 26:Suppl 2. S199–S201.

8. Joo K, Park W, Lim MJ, Kwon SR, Yoon J. Serum procalcitonin for differentiating bacterial infection from disease flares in patients with autoimmune diseases. J Korean Med Sci. 2011; 26:1147–1151.

9. Buhaescu I, Yood RA, Izzedine H. Serum procalcitonin in systemic autoimmune diseases--where are we now? Semin Arthritis Rheum. 2010; 40:176–183.

10. Wang C, Zhong D, Liao Q, Kong L, Liu A, Xiao H. Procalcitonin levels in fresh serum and fresh synovial fluid for the differential diagnosis of knee septic arthritis from rheumatoid arthritis, osteoarthritis and gouty arthritis. Exp Ther Med. 2014; 8:1075–1080.

11. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977; 20:895–900.

12. Gupta D, Agarwal R, Aggarwal AN, Singh N, Mishra N, Khilnani GC, et al. Guidelines for diagnosis and management of community-and hospital-acquired pneumonia in adults: Joint ICS/NCCP(I) recommendations. Lung India. 2012; 29:Suppl 2. S27–S62.

13. Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012; 55:1279–1282.

14. Trowbridge RL, Rutkowski NK, Shojania KG. Does this patient have acute cholecystitis? JAMA. 2003; 289:80–86.

15. Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, et al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2013; 20:24–34.
16. Woodford HJ, George J. Diagnosis and management of urinary infections in older people. Clin Med (Lond). 2011; 11:80–83.

18. Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001; 32:331–351.

19. Dieppe P, Swan A. Identification of crystals in synovial fluid. Ann Rheum Dis. 1999; 58:261–263.

20. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012; 64:1431–1446.

21. Christ-Crain M, Müller B. Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007; 30:556–573.

22. Chan T, Gu F. Early diagnosis of sepsis using serum biomarkers. Expert Rev Mol Diagn. 2011; 11:487–496.

23. Pfäfflin A, Schleicher E. Inflammation markers in point-of-care testing (POCT). Anal Bioanal Chem. 2009; 393:1473–1480.

24. Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998; 24:888–889.

25. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011; 171:1322–1331.

26. Tsalik EL, Jaggers LB, Glickman SW, Langley RJ, van Velkinburgh JC, Park LP, et al. Discriminative value of inflammatory biomarkers for suspected sepsis. J Emerg Med. 2012; 43:97–106.

27. Pavcnik-Arnol M, Hojker S, Derganc M. Lipopolysaccharide-binding protein, lipopolysaccharide, and soluble CD14 in sepsis of critically ill neonates and children. Intensive Care Med. 2007; 33:1025–1032.

28. Liu W, Sigdel KR, Wang Y, Su Q, Huang Y, Zhang YL, et al. High level serum procalcitonin associated gouty arthritis susceptibility: from a southern chinese Han population. PLoS One. 2015; 10:e0132855.

29. Martinon F. Mechanisms of uric acid crystal-mediated autoinflammation. Immunol Rev. 2010; 233:218–232.

30. Dalbeth N, Haskard DO. Mechanisms of inflammation in gout. Rheumatology (Oxford). 2005; 44:1090–1096.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download