Abstract
Purpose
Erlotinib-gemcitabine combined chemotherapy is considered as the standard treatment for unresectable pancreatic cancer. This study aimed to determine the clinical factors associated with response to this treatment.
Materials and Methods
This retrospective study included 180 patients with unresectable pancreatic cancer who received ≥2 cycles of gemcitabine-erlotinib combination therapy as first-line palliative chemotherapy between 2006 and 2014. "Long-term response" was defined as tumor stabilization after >6 chemotherapy cycles.
Results
The median progression-free survival (PFS) and overall survival (OS) were 3.9 and 8.1 months, respectively. On univariate analysis, liver metastasis (p=0.023) was negatively correlated with long-term response. Locally advanced stage (p=0.017), a history of statin treatment (p=0.01), and carcinoembryonic antigen levels <4.5 (p=0.029) had a favorable effect on long-term response. On multivariate analysis, a history of statin treatment was the only independent favorable factor for long-term response (p=0.017). Prognostic factors for OS and PFS were significantly correlated with liver metastasis (p=0.031 and 0.013, respectively). A history of statin treatment was also significantly associated with OS after adjusting for all potential confounders (hazard ratio, 0.48; 95% confidence interval, 0.26–0.92; p=0.026).
Pancreatic cancer is one of the leading causes of cancer death worldwide. Despite recent advances in therapy, overall survival (OS) is less than 6 months, and 5-year survival rates for unresectable pancreatic cancer patients are very low.12 Although gemcitabine was originally regarded as the standard chemotherapy agent for advanced pancreatic cancer, any benefit was marginal, with an objective response rate of only 5% and a median survival rate of 5.7 months.3 Throughout the last 20 years, a number of clinical trials with various combinations of anti-cancer drugs have been performed. Combination chemotherapy with folinic acid, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) and gemcitabine/nab-paclitaxel have shown a remarkable improvement in survival benefits for advanced metastatic pancreatic cancer.45 However, the OS for both regimens is still less than 1 year, and they cannot be prescribed to all patients with unresectable pancreatic cancer, due to their relatively severe toxicity profiles.
Owing to increased information on the molecular changes in cancer, cell-specific anti-cancer targeted agents have been introduced into clinical practice. This shift in the treatment paradigm is focused on magnifying the anti-tumor effects while minimizing off-target adverse effects, which are the most common concern in patient management. There have been several clinical trials to assess the benefits of targeted agents including erlotinib, bevacizumab, and cetuximab for pancreatic cancer. To date, only erlotinib has been accepted as a standard drug when combined with gemcitabine.6 However, there are several issues with using gemcitabine-erlotinib combination chemotherapy for advanced pancreatic cancer, the most important being that there is no predictive factor for response to these drugs. In lung cancer, there are several well-known genetic predictive factors for response to tyrosine kinase inhibitors. 78 Thus far, most studies have concentrated on the relationship between genetic differences and response to erlotinib. However, it is also necessary to examine the clinical profiles of advanced pancreatic cancer patients, as they usually have coexisting diseases or conditions that require medications such as metformin or statins.9 In particular, statins have been evaluated for their potential anti-tumor effects. Recently, Nielsen, et al.10 reported that statin use in cancer patients is associated with reduced cancer-related mortality in 13 cancer types. Furthermore, several preclinical studies have suggested that statins have potential anti-cancer properties, including inhibition of cell proliferation, resulting in inhibition of tumor growth and angiogenesis.11121314 Other studies have demonstrated that the anticancer effect is due to statin-induced posttranscriptional modification of Ras and RhoA.151617
In this study, we attempted to determine the clinical factors that predict response to gemcitabine-erlotinib combination chemotherapy in advanced pancreatic cancer patients. We focused on the effect of concomitant statins on the oncologic outcomes of patients who received gemcitabine-erlotinib chemotherapy.
This study included patients with unresectable or recurrent pancreatic cancer who received gemcitabine plus erlotinib as first-line chemotherapy at Severance Hospital (Seoul, Korea), between November 2006 and January 2014. All patients were histologically diagnosed with pancreatic adenocarcinoma and underwent dynamic computed tomography (CT) of the abdomen and pelvis. The tumors were classified as recurrent, locally advanced (including stage III), and advanced pancreatic cancer (including stage IV) using the American Joint Committee on Cancer (7th edition) guidelines. Patients were excluded if they had other malignancies, were unable to determine treatment response, had serum creatinine or total bilirubin levels >1.5×upper limit of normal (ULN), or had cardiomegaly on a chest radiograph.
The clinical factors examined in this study were sex; age; history of diabetes mellitus, smoking, or chronic pancreatitis; type of operation; location or size of tumor; involvement of regional lymph nodes; number of metastatic organs; location of metastasis; locally advanced stage; involvement of major vessels; laboratory parameters including carbohydrate antigen (CA) 19-9, carcinoembryonic antigen (CEA), and bilirubin levels; and history of statin or aspirin treatment. Statins and aspirin could have been taken before or simultaneously with chemotherapy. Levels of CA 19-9, CEA, and bilirubin were evaluated before the first round of chemotherapy.
Erlotinib was administered at a dose of 100 mg daily without interruption. Gemcitabine (1000 mg/m2) was administered by a 30-minute intravenous infusion on days 1, 8, and 15. The cycles were repeated every 28 days, provided that the absolute neutrophil count was >1500/µL, the platelet count was >100000/µL, bilirubin was <2×ULN, and serum creatinine was <1.5×ULN. Treatment was discontinued in cases of progressive disease, unacceptable adverse events, or withdrawal of patient consent. Tumor responses were assessed via CT every 8 weeks (two cycles) subject to the patient's condition or earlier in those with suspected progression based on the Response Evaluation Criteria in Solid Tumors.
The primary goal was to determine any clinical factors that influenced the response to gemcitabine-erlotinib chemotherapy. Therefore, we defined "long-term response" as occurring when gemcitabine-erlotinib chemotherapy was proven to show a tumor stabilization effect after six cycles of chemotherapy. The parameters for evaluating the tumor stabilization effect included complete response (CR), partial response (PR), and stable disease (SD) rates. In addition, we analyzed progression-free survival (PFS) and OS rates.
We reviewed the drug name, the cumulative duration of statin use, and the cumulative dose of statin intake. Statin users were defined as patients who received statin medications for at least 30 days. The statins prescribed during the study period were atorvastatin, rosuvastatin, simvastatin, and pitavastatin. The brand names of statins used in this study were as follows: simvastatin (Cholesnone, Simvastar, Vytorin); atorvastatin (Atorva, LipiLOU, Lipitor); rosuvastatin (Crestor, Vivacor); pitavastatin (Livalo).
The cumulative dose was standardized for different statins using the defined daily doses (DDDs) recommended by the World Health Organization.1018 The DDD for the 30 mg formulation of simvastatin was used as a reference, and the DDDs for the other statins were used to convert each statin dose to a dose equivalent to 30 mg of simvastatin.
The clinical factors associated with response rates were investigated by using multivariable regression modeling. The influence of potential prognostic factors on OS and PFS was assessed by using the Cox hazards regression model to estimate the hazard ratio (HR). Values of p<0.05 were considered to be statistically significant. Statistical analysis was performed using SPSS software, version 21.0 (SPSS Inc., Chicago, IL, USA).
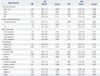
This study included 180 patients with unresectable pancreatic cancer who received at least 2 cycles of gemcitabine-erlotinib combination therapy as first-line palliative chemotherapy between November 2006 and January 2014. The characteristics of the patients are shown in Table 1. The median age was 65 years. Twenty-four of 180 patients (13.3%) had locally advanced disease, and 156 (86.7%) had metastatic disease or recurrent disease after curative resection. The metastasis had spread to at least two organs in 61 patients (33.9%) and to one organ in 95 (52.8%). The liver was the most frequently metastatic site in 122 patients (67.8%), and carcinomatosis occurred in 39 (21.7%). Pretreatment CA 19-9 levels >1000 U/mL were detected in 87 patients (50.3%), and the median level was 1020 U/mL. Pretreatment CEA levels >4.5 ng/mL were measured in 84 patients (50.9%), and the median level was 4.67 ng/mL. The median number of gemcitabine-erlotinib combination chemotherapy cycles was four.
An objective response after two cycles of chemotherapy was observed in 40 patients (0 CR and 40 PR), resulting in a response rate of 22.2%. The disease control rate was 63.3% after two cycles of chemotherapy. A "long-term response," which was defined as having achieved CR, PR, or SD after six cycles of chemotherapy, was observed in 54 patients (30%). The median PFS and OS were 3.9 and 8.1 months, respectively. At the time of final data analysis, 162 of 180 patients (90%) had died.
Among all 180 patients, 17 received statin. Atorvastatin was most commonly prescribed (n=8, 47.1%), followed by rosuvastatin (n=6, 35.3%), simvastatin (n=2, 11.8%), and pitavastatin (n=1, 5.8%), as described in Supplementary Table 1 (only online). Among the patients that received statins during the study period, the median duration of use was 182 days.
Univariate analyses of the clinical characteristics indicated that six different factors were associated with response after two cycles of chemotherapy (Table 2). The following clinical factors showed statistical significance: pancreatic tail cancer [odds ratio (OR), 0.31; 95% confidence interval (CI), 0.12–0.78; p=0.013], locally advanced stage (OR, 3.71; 95% CI, 1.5–9.1; p=0.004), one metastatic organ (OR, 0.36; 95% CI, 0.14–0.91; p=0.03), two or more metastatic organs (OR, 0.15; 95% CI, 0.05–0.47; p=0.001), carcinomatosis (OR, 0.15; 95% CI, 0.05–0.47; p=0.01), and a history of statin treatment (OR, 4.79; 95% CI, 1.71–13.40; p=0.003).
In the multivariate logistic regression model, which included six statistically significant clinical factors from the univariate model, three factors showed independent statistical significance (Table 2). The presence of one metastatic organ (OR, 0.30; 95% CI, 0.11–0.81; p=0.017) and carcinomatosis (OR, 0.17; 95% CI, 0.02–1.00; p=0.05) were unfavorable factors for response. A history of statin treatment was a favorable factor for response after two cycles of chemotherapy (OR, 4.69; 95% CI, 1.41–15.6; p=0.012).
We used univariate and multivariate analyses to determine factors associated with "long-term response." Four different factors had an effect on "long-term response" in univariate analysis (Table 2). These included locally advanced stage (OR, 2.71; 95% CI, 1.13–6.51; p=0.025), liver metastasis (OR, 0.46; 95% CI, 0.24–0.89; p=0.023), history of statin treatment (OR, 3.86; 95% CI, 1.38–10.78; p=0.01), and CEA level <4.5 ng/mL (OR, 2.15; 95% CI, 1.08–4.29; p=0.029). Multivariate analysis identified only one clinical feature that may affect "long-term response": a history of statin treatment, which was an independent favorable factor (OR, 4.11; 95% CI, 1.28–13.2; p=0.017).
According to the Cox hazards regression model, liver metastasis was a prognostic factor that significantly affected OS (HR, 1.78; 95% CI, 1.05–3.01; p=0.031) and PFS (HR, 1.55; 95% CI, 1.09–2.19; p=0.013) (Table 3). A history of statin treatment, which was revealed to be the only favorable factor associated with "long-term response," was also significantly associated with OS after adjusting for all potential confounders (HR, 0.48; 95% CI, 0.26–0.92; p=0.026) (Fig. 1, Table 3).
Pancreatic cancer has a poor outcome, with a 5-year survival rate of only 5%.123 There have been significant medical advances, including targeted agents for unresectable advanced pancreatic cancer; however, the median survival time is approximately 6 months after the standard chemotherapy regimen of gemcitabine combined with erlotinib.6 Because of this very poor prognosis, a favorable response in the early chemotherapy cycles is important for longer survival times.
In this study, statins in combination with erlotinib and gemcitabine chemotherapy were associated with a good response in advanced pancreatic cancer for both short- and long-term chemotherapy. It is notable that concomitant use of statins showed a positive, long-term effect after >6 cycles of gemcitabine-erlotinib treatment. Considering that the median survival time of patients with advanced pancreatic cancer was approximately 6 months, the use of statins as chemoadjuvant treatment was meaningful in that it maintained tumor stabilization for the median survival time, assuming that the duration of one cycle of chemotherapy was equivalent to 1 month.
Many clinical studies have evaluated the potential anti-cancer effects of additional statin treatment. Colon cancer has been widely studied for associations between statin use and cancer incidence.192021222324 The protective effect of statins against the development of pancreatic cancer has also been presented.25 Khurana, et al.25 reported that statin administration for >6 months was associated with a reduced risk of pancreatic cancer by 67%. Statins are 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors that reduce the synthesis of intracellular cholesterol by reversible inhibition of the conversion of HMG-CoA to mevalonate in the mevalonate pathway.26 This pathway also produces farnesyl pyrophosphate and geranylgeranyl pyrophosphate, both of which are involved in the posttranslational modification of cellular proteins including the Ras, Rab, Rac, and Rho families, which in turn influence cell proliferation, cell motility, and posttranslational modification. 2728 Statins block this pathway downstream, which may result in anti-cancer activity due to inhibition of tumor growth, angiogenesis, and metastasis.1214151629
Hong, et al.30 reported the first human clinical trial with statins used in combination with standard gemcitabine chemotherapy for advanced pancreatic cancer. This study showed that adding low-dose simvastatin to gemcitabine does not provide any clinical benefit when compared with gemcitabine plus a placebo.
However, we need to consider the potential synergic effect of statins and erlotinib referred to in many studies. By depleting mevalonate metabolites such as dolichol, farnesyl, and geranylgeranyl pyrophosphate through the inhibition of HMG-CoA reductase, statins have the potential to inhibit both epidermal growth factor receptor (EGFR) and its downstream signaling cascades.313233 Mantha, et al.34 reported that a combination of lovastatin and gefitinib, an anti-EGFR agent, showed significant synergistic anti-cancerous effects and enhanced EGFR inhibition.
Approximately 70–90% of pancreatic cancer patients are thought to have the v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).35 It is known that KRAS mutations are associated with a worse prognosis after anti-EGFR-targeted therapy in lung and colorectal cancer. It has been reported that statins may overcome resistance to anti-EGFR-targeted agents in colon cancer cells with the KRAS mutation.36 Our study also demonstrated that statins in combination with erlotinib were positively associated with a "long-term response." Therefore, statins may be a potential chemoadjuvant therapy along with anti-EGFR treatment for advanced pancreatic cancer patients with KRAS mutations.
In summary, this study suggests that statins have a favorable effect on the response to gemcitabine and erlotinib chemotherapy in advanced pancreatic cancer after six cycles of chemotherapy. As the median survival time of patients with advanced pancreatic cancer was approximately 6 months, statins may have a significant role as chemoadjuvant therapy for stabilizing long-term tumor growth. It is also important to note that statins have been used for a long time in cardiovascular disease with a proven safety record. Furthermore, prospective studies are needed to evaluate the efficacy of statins combined with chemotherapy containing anti-EGFR-targeted agents to overcome resistance to this treatment in advanced pancreatic cancer with KRAS mutations.
Figures and Tables
 | Fig. 1A history of statin treatment was revealed to be the only favorable factor associated with "long-term response" on multivariate analysis and with OS. Kaplan-Meier curves for overall survival in pancreatic cancer patients with statin and without statin (log-rank p=0.059). OS, overall survival. |
Table 1
Baseline Patient Characteristics

LN, lymph node; IQR, interquartile range (25–75%); CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CR, complete response; PR, partial response; SD, stable disease; PFS, progression-free survival; OS, overall survival; CI, confidence interval; PPPD, pylorus-preserving pancreaticoduodenectomy.
*When gemcitabine-erlotinib chemotherapy was proven to show a tumor stabilization effect (CR, PR, or SD) by six cycles of chemotherapy.
Table 2
Response Factor Analysis

Table 3
Prognosis Factor Analysis
References
3. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15:2403–2413.

4. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364:1817–1825.

5. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013; 369:1691–1703.

6. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25:1960–1966.

7. Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008; 9:962–972.

8. Murray S, Dahabreh IJ, Linardou H, Manoloukos M, Bafaloukos D, Kosmidis P. Somatic mutations of the tyrosine kinase domain of epidermal growth factor receptor and tyrosine kinase inhibitor response to TKIs in non-small cell lung cancer: an analytical database. J Thorac Oncol. 2008; 3:832–839.

9. Suh Y, Kim BK, Shin DH, Kim JS, Ko YG, Choi D, et al. Impact of statin treatment on strut coverage after drug-eluting stent implantation. Yonsei Med J. 2015; 56:45–52.

10. Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012; 367:1792–1802.

11. Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003; 9:10–19.
12. Dimitroulakos J, Marhin WH, Tokunaga J, Irish J, Gullane P, Penn LZ, et al. Microarray and biochemical analysis of lovastatin-induced apoptosis of squamous cell carcinomas. Neoplasia. 2002; 4:337–346.

13. Jakobisiak M, Golab J. Potential antitumor effects of statins (Review). Int J Oncol. 2003; 23:1055–1069.

14. Park HJ, Kong D, Iruela-Arispe L, Begley U, Tang D, Galper JB. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ Res. 2002; 91:143–150.

15. Lee J, Lee I, Park C, Kang WK. Lovastatin-induced RhoA modulation and its effect on senescence in prostate cancer cells. Biochem Biophys Res Commun. 2006; 339:748–754.

16. Park C, Lee I, Kang WK. Lovastatin-induced E2F-1 modulation and its effect on prostate cancer cell death. Carcinogenesis. 2001; 22:1727–1731.

17. Winiarska M, Bil J, Wilczek E, Wilczynski GM, Lekka M, Engelberts PJ, et al. Statins impair antitumor effects of rituximab by inducing conformational changes of CD20. PLoS Med. 2008; 5:e64.

18. Wertheimer AI. The defined daily dose system (DDD) for drug utilization review. Hosp Pharm. 1986; 21:233–234. 239–241. 258
19. Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal adenomatous polyps. Int J Colorectal Dis. 2013; 28:469–476.

20. Hoffmeister M, Chang-Claude J, Brenner H. Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: a population-based case-control study. Int J Cancer. 2007; 121:1325–1330.

21. Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005; 352:2184–2192.

22. Robertson DJ, Riis AH, Friis S, Pedersen L, Baron JA, Sørensen HT. Neither long-term statin use nor atherosclerotic disease is associated with risk of colorectal cancer. Clin Gastroenterol Hepatol. 2010; 8:1056–1061.

23. Samadder NJ, Mukherjee B, Huang SC, Ahn J, Rennert HS, Greenson JK, et al. Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer. 2011; 117:1640–1648.

24. Simon MS, Rosenberg CA, Rodabough RJ, Greenland P, Ockene I, Roy HK, et al. Prospective analysis of association between use of statins or other lipid-lowering agents and colorectal cancer risk. Ann Epidemiol. 2012; 22:17–27.

25. Khurana V, Sheth A, Caldito G, Barkin JS. Statins reduce the risk of pancreatic cancer in humans: a case-control study of half a million veterans. Pancreas. 2007; 34:260–265.
27. Pruitt K, Der CJ. Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett. 2001; 171:1–10.

28. Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002; 8:23–30.

29. Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, et al. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology. 2002; 122:308–317.

30. Hong JY, Nam EM, Lee J, Park JO, Lee SC, Song SY, et al. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother Pharmacol. 2014; 73:125–130.

31. Carlberg M, Dricu A, Blegen H, Wang M, Hjertman M, Zickert P, et al. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface. Evidence for a new link between 3-hydroxy-3-methylglutaryl-coenzyme a reductase and cell growth. J Biol Chem. 1996; 271:17453–17462.

32. Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001; 20:1594–1600.

33. Gibbs JB, Oliff A, Kohl NE. Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic. Cell. 1994; 77:175–178.

34. Mantha AJ, Hanson JE, Goss G, Lagarde AE, Lorimer IA, Dimitroulakos J. Targeting the mevalonate pathway inhibits the function of the epidermal growth factor receptor. Clin Cancer Res. 2005; 11:2398–2407.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download