Abstract
Purpose
The systemic inflammation biomarker, Neutrophil-to-Lymphocyte Ratio (NLR), has been reported as one of the adverse prognostic factors for hepatocellular carcinoma (HCC) patient. The purpose of this study was to evaluate whether NLR could predict the risk of recurrence and death for the HCC patient, according to Milan criteria after hepatectomy.
Materials and Methods
Retrospective analysis was performed on a database of HCC patients who underwent hepatectomy between March 2001 and December 2011. The cutoff value of NLR was decided by receiver operating characteristic (ROC) curve analysis. Univariate and multivariate regression analyses were performed to identify predictive factors of recurrence and death.
Results
A total of 213 patients were included in the present study. The median follow-up period was 48 months. One hundred and seven patients were experienced tumor recurrence; forty of them recurred within 12 months (early recurrence). NLR ≥1.505, albumin ≤3.75 g/dL, microvascular invasion and high grade of cirrhosis were found to be independent factors for adverse recurrence-free survival in multivariate regression analysis. And NLR ≥1.945 was also found as a prognosis factor for early recurrence by univariate regression analysis.
Hepatocellular carcinoma (HCC) is the third common leading cancer in Korea.1 And hepatectomy is considered to be the gold standard treatment for the patients who preserved liver function. Recently, many of HCC patients are diagnosed in the state of Child-Pugh grade A due to the advance in diagnosis and the efficient and organized application of nationwide surveillance program in Korea.23 Even the overall survival (OS) is satisfied, nevertheless, the high incidence of tumor recurrence remains a challenge.1 And the OS was mainly influenced by tumor recurrence. Minagawa, et al.4 reported that the OS after second hepatectomy was siginificantly poor in patients whose cancer recurred within 12 months (early recurrence) after first hepatectomy compared to those whose cancer recurred more than 12 months (late recurrence) after initial operation. From that point of view, disease-free survival is an important prognostic factor for survival. The mechanism of high incidence of recurrence for HCC patients and the risk factors leading to early recurrence have not been clearly defined.
An increased evidence of relationship between systemic inflammation and tumor biology has been reported. Neutrophil-to-Lymphocyte Ratio (NLR) was considered as a reliable parameter for monitoring and evaluation of systemic inflammatory response.5 And NLR is easily and repeatedly obtained from peripheral blood. Up to date, NLR has been investigated for its prognostic role in HCC after curative resection,678 transcatheter arterial chemoembolization (TACE),9 radiofrequency ablation (RFA),10 liver transplantation (LT),111213 hepatic arterial infusion chemotherapy,14 and sorafenib monotherapy.15 Since the prognostic value of NLR at Milan criteria level was not inclusive, we assessed the impact of elevated NLR on the outcomes of HCC patients, confirming to Milan criteria after initial hepatectomy during a long follow-up period.
Between March 2001 and December 2011, a total of 621 patients with HCC underwent hepatectomy at the Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Ajou University School of Medicine, Suwon, Korea. The inclusion and exclusion criteria were as follows: 1) Milan criteria (based on radiological imaging): solitary tumor ≤5 cm in diameter and up to three nodules ≤3 cm in diameter; without any invasion into the major portal/hepatic vein branches and no extrahepatic metastasis. 2) Primary treatment was surgery resection. Patients with preoperative therapy, such as TACE, RFA, second surgical resection or LT for recurrence were excluded. 3) Patents who had clinical evidence of infection or white blood cell count more than >10×109/L, immune system disease, hematology disease, used hematology influenced drugs within 1 month and patients with other cancer were excluded from this study. Finally, two hundred and thirteen patients which consisted of 166 males and 47 females with a median age of 53 years (range 20–79 years) were enrolled in this study. Serum hepatitis B surface antigen (HBsAg) was positive in 162 cases, and anti-Hepatitis C viral antibody in 15 cases.
Clinicopathologic characteristics of enrolled patients including gender, age, etiology of cirrhosis, comorbidities [diabetes mellitus (DM), hypertension], surgical procedure (anatomical resection and non-anatomical resection), albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), indocyanine green retention rate at 15 min (ICG R15), alpha-fetoprotein (AFP), tumor size, intrahepatic metastasis, microvascular invasion, microscopic resection margin, capsular invasion and tumor recurrence were collected and detailed in Table 1. In our institute, the procedures of hepatectomy were as follows: anatomical resection: right/left hemihepatectomy or central bisectionectomy (31 cases, 14.6%), left lateral sectionectomy, right anterior/posterior sectionectomy (42 cases, 19.7%) and one segmentectomy (43 cases, 20.2%). Non-anatomical resection: wedge resection (97 cases, 45.5%). All data were retrospectively analyzed from the database of our institute which was prospectively collected.
The median follow up period was 48 months (range 1 to 156 months). And the clinical follow-up strategy followed a strict and stable protocol. All patients in our institute were screened monthly during the first 6 months after surgery for tumor marker such as AFP and laboratory data such as complete blood count (CBC), AST, ALT, albumin, total bilirubin. An enhanced computed tomography scan was performed at 6-month intervals. If recurrence was suspected, additional investigations such as magnetic resonance imaging or positron emission tomography-computed tomography were performed.
The recurrent pattern was defined as early recurrence (recurred within 12 months after initial hepatectomy) and late recurrence (recurred beyond 12 months after initial hepatectomy).
Five types of treatment were adopted to treat the tumor recurrence after initial hepatectomy, such as re-resection, TACE, RFA, salvage LT and conservative management. Finally, fourteen patients with good future liver function were eligible for repeat resection; twenty eight patients received RFA; forty two patients received TACE; ten patients who with multi recurrence tumor and/or stayed at end stage of liver cirrhosis disease received salvage LT; and thirteen patients were received conservative management. The treatment methods for recurrence tumor are listed in Fig. 1.
CBC and blood biochemical examination were carried out in all patients within 4 days before operation. The NLR was calculated from the differential ratios by dividing neutrophil to lymphocyte ratio in CBC. In the present study an receiver operating characteristic (ROC) curve indicated that the value of 1.505 was the best cutoff value of NLR for recurrence after hepatectomy. The area under the curve (AUC) was 0.643. The p value was 0.005 (Fig. 2A). The optimal cutoff values of albumin, AST and tumor size for patients overall recurrence-free survival (RFS) after hepatectomy were 4.15 g/L (AUC=0.648, p=0.000), 36.5 U/L (AUC=0.600, p=0.012), and 2.65 cm (AUC= 0.627, p=0.001), respectively. In the 107 recurrence patients, the value of 1.945 determined by ROC curve was the best cutoff value of NLR for early recurrence; the AUC was 0.643, and the p value was 0.005 (Fig. 2B).
The total patients were divided into two groups by optimal cutoff value of NLR (1.505); normal NLR group and elevated NLR group. There were no difference between the two groups in the characteristics, such as gender, age, etiology of cirrhosis, hypertension, non-anatomical resection, albumin, total bilirubin, AST, ALT, ICG R15, AFP, tumor size, microvascular invasion and capsular invasion. As expected, elevated NLR group had a more tumor recurrence patients, as indicated by more patients with intrahepatic metastasis. More patients with DM were found in the elevated NLR group (Table 1).
The recurrent patients were divided into two subgroups by recurrence time: early recurrence group (recurred within 12 months) and late recurrence group (recurred beyond 12 months). The OS was significantly different between the early and late recurrence group (p=0.000). The early recurrence group had relatively more patients with elevated NLR, intrahepatic metastasis, microvascular invasion, high grade of cirrhosis (grade 4) and serum HBsAg-positive. On the contrary, the late recurrence group had more patients with high level of AST and significantly longer OS (Table 2).
Continuous variables were expressed as mean±standard deviation and compared using Student t test. Categorical data were presented as frequency and analyzed by using the Fisher χ2 test. Multivariate analysis was performed with Cox regression for significant variables on univariate analysis. The Kaplan-Meier method was used to analyses the OS and RFS, and compared using the log-rank test. All statistical analyses were performed with the statistical package for the social sciences (SPSS) 19.0 (SPSS Inc., Chicago, IL, USA). Confidence intervals (CI) were constructed at 95%, and p value <0.05 was considered statistically significant.
The 1, 3, and 5 year cumulative OS was 95.2%, 86.9%, and 81.3%, respectively (Fig. 3A) whereas the 1, 3, and 5 year cumulative RFS was 79.9%, 60.5%, and 50.1%, respectively (Fig. 3B). The total patients were divided into two groups by optimal cutoff value of NLR (1.505); normal NLR group (NLR<1.505) and elevated NLR group (NLR≥1.505). The RFS time of elevated NLR group was significantly shorter than that of the normal NLR group (the log-rank test, p=0.010) (Fig. 3C). The 1, 3, and 5 year of RFS in normal NLR group was 88.9%, 69.8%, and 57.0%, respectively. In contrast, the 1, 3, and 5 year of RFS in elevated NLR group was 74.0%, 53.5%, and 39.9%, respectively. However, there was no difference in OS between the two groups (the logrank test, p=0.114) (Fig. 3D).
In this cohort study, there were 107 patients suffered from tumor recurrence. The patients who experienced early recurrence had a poor OS (the log-rank test, p=0.000) (Fig. 4). The 1, 3, and 5 year of cumulative OS for the early recurrence group was 78.9%, 46.4%, and 42.5%, respectively. In contrast, the 1, 3, and 5 year of OS for the late recurrence group was 98.5%, 97.0%, and 86.8%, respectively.
The prognosis factors for RFS and OS of HCC after surgical resection were examined based on the following clinicopathological variables: etiology of cirrhosis (hepatitis B virus, hepatitis C virus), co-morbidities (DM, hypertension), elevated NLR (>1.505), low ablumin (<4.15 g/L), elevated AST (>36.5 U/L), large tumor size (>2.95 cm), multiple tumor number, non-anatomical resection, microvascular invasion, portal vein invasion, intrahepatic metastasis, capsular invasion, high cirrhosis grade (grade 3 and 4),16 and high grade of Edmondson-Steiner (ES; grade 3 and 4).17 The variables of elevated NLR, low ablumin, elevated AST, large tumor size, multiple tumor number, microvascular invasion, portal vein invasion, intrahepatic metastasis and high cirrhosis grade which were significant predictors of RFS analyzed by univariate regression analysis were considered in multivariate regression analysis. And the variables of elevated NLR, microvascular invasion and cirrhosis grade were significant predictors of RFS (Table 3). However, NLR failed to predict OS time analyzed by univariate regression. Univariate regression analysis found that DM, low ablumin, elevated AST, multiple tumor number, microvascular invasion, portal vein invasion and intrahepatic metastasis were significant risk factors for OS. Unfortunately, no risk factor was found by multivariate analysis of OS (Table 4).
The elevated NLR (1.945), combined with multiple tumor number, mircovascular invasion, portal vein invasion and intrahepatic metastasis were considered as risk factors for early recurrence, when determined by univariate regression analysis. On the other hand, however, microvascular invasion was the only risk factor for early recurrence, when determined by multivatiate regression analysis (Table 5).
One and half century ago, the connection of inflammation and malignancy was observed by Rudolf Virchow for the first time18 in leucocytes in neoplastic tissues. An increased comprehension of the relationship between inflammation and cancer was made by experimental and clinical research. Inflammation plays an important role in the development and progression of cancer, including initiation, promotion, malignant conversion, invasion, and metastasis,19 and NLR was considered as a reliable parameter for monitoring and evaluation of systemic inflammatory response.5 Fortunately, NLR could repeatedly and easily be obtained from peripheral blood without costly expenditure. More recently, an increasing number of studies have shown that elevated NLR has adverse prognosis on recurrence and OS for HCC patients after different treatment procedures, such as curative resection,678 TACE,9 RFA10 and LT,111213 hepatic arterial infusion chemotherapy,14 and sorafenib monotherapy.15 In the present study, we further demonstrated that elevated NLR was a prognostic factor of tumor recurrence and early recurrence for HCC patients who meet the Milan criteria after surgical resection, and the optimal cutoff value was 1.505 and 1.945, respectively, calculated by ROC curve (1.505, AUC=0.643, p=0.006; 1.945, AUC=0.641, p=0.015) (Fig. 2).
The underlying mechanism in the association of elevated NLR and adverse outcomes of HCC patients is not clearly defined: recent clinical and experimental researches shed some new light onto their roles. Mano, et al.7 demonstrated that accumulation of tumor-associated macrophages (TAMs) in the HCC lesion is associated with a high NLR after curative resection. TAMs have been reported to be a major component of the tumor inflammatory microenvironment and promote cancer initiation and malignant progression.20 Human TAMs can express various pro-angiogenic factors in tumors, including vascular endothelial growth factor (VEGF)21 and thymidine phosphorylase.22 Peng, et al.23 reported that the count of TAMs was closely related to micro-vessel density in HCC, suggesting that TAMS have a link with HCC angiogenesis and metastasis. The Interleukin (IL)-6 and IL-8 cytokines which are expressed by TAMs in tumors may promote systemic neutrophilia.242526 Mano, et al.7 and Motomura, et al.12 reported a correlation between elevated NLR and upregulation of IL-17 production in peritumoral regions of the liver. The proinflammatory cytokine IL-17 has been demonstrated to foster tumor immune escape27 and promote tumor growth in HCC.28 IL-17 could be indirectly upregulated by promoted expansion of IL-17-producing CD8+ T cell by TAMs in HCC patients.29 In summary, TAMs plays an important role for tumor progression, and NLR could indirectly reflect in peripheral blood of TAMS through the cytokines such as IL-6, IL-8, and IL-17.
In this cohort study, the median follow-up period was 48 months (range 1 to 156 months), and the cumulative 1, 3, and 5 year OS was 95.2%, 86.9%, and 81.4%, respectively (Fig. 3). However, the RFS remains unsatisfactory. Total 107 patients experienced tumor recurrence. The cumulative 1, 3, and 5 year RFS was 86.1%, 68.7%, and 44.2%, respectively (Fig. 3). When patients were divided into two groups by optimal cutoff value of NLR (1.505), and the elevated NLR group was found to have more tumor recurrence patients (p=0.000) and more patients with intrahepatic metastasis (p=0.014). As expected the variable of elevated NLR (1.505) together with microvascular invasion, low albumin, and high grade of cirrhosis were found to be significant predictors for RFS analyzed by multivariate regression analysis (Table 3). Our present finding is consistent with previous reports of Sumie, et al.30 and Poon, et al.31 that microvascular invasion and cirrhosis were independent risk factors for tumor recurrence.
Minagawa, et al.4 reported that early recurrence patients whose cancer recurred within 12 months after initial hepatectomy have a worse OS than late recurrence, it is believed that intrahepatic metastasis represents early recurrence and is associated with vascular invasion and subsequent intrahepatic venous spread.32 Similar outcome was also found in our present study. In our experience of 40 early recurrence patient treatments, 90% of (36/40) cases developed to multiple recurrence and the outcome was unsatisfactory even when treated by salvage LT. The OS of early recurrence patients was significantly worse than the late recurrence (p=0.000). The NLR was found to be an independent risk factor [hazard ratio (HR), 1.678, p=0.004] for early recurrence in univariate regression analysis with 1.945 cutoff value of NLR (Table 5). As mentioned above, elevated NLR showed a strong relationship with tumor progression: underlying mechanism might be that angiogenesis and metastasis of the tumor cell in the tumor lesion are promoted by TAMs.
In our present study, more patients with DM were found in elevated NLR group. DM is considered to be a risk factor for OS by univariate analysis but it failed by multivariate analysis. The relationship between systematic inflammatory and DM has been proved by earlier studies33 that systematic inflammation may induce insulin resistance and decrease insulin secretion. However, a question of whether DM is an adverse factor for HCC remains controversial. Two resent studies3435 identified that DM is an independent factor for poor survival in HCC patients who underwent curative therapy. On the contrary, however, Poon, et al.36 reported that DM did not seem to influence either the risk of recurrence or long term OS after resection of HCC.
In the present study, NLR failed to predict for prognosis for OS of the HCC patients confirming to Milan criteria after surgical resection. The potential reasons maybe that the inclusion criterion of this study was Milan criteria and the patients preserved liver function well. The primary good liver function would facility to choose an optimal treatment for tumor recurrence. Fig. 1 shows the types of treatment for tumor recurrence patients. Fourteen patients received repeated resection and the remaining patients who were not suitable for resection received TACE, RFA, systemic chemotherapy, oral chemotherapy, supportive treatment and/or salvage LT. The 1, 3, 5-year OSs of repeated resection were 100%, 92.3%, and 92.3%, respectively. However, the 1, 3, 5-year OS of the remaining patients were 91.2%, 78.1%, and 67.5%, respectively (p=0.022). Therefore the repeated resection seems to significantly prolong the OS of HCC recurrent patients and 8 of 14 patients belonged to the elevated NLR group.
In conclusion, the inflammatory biomarker of preoperative elevated NLR indirectly reflect vascular infiltration factors in the tumor microenvironment, and preoperative elevated NLR is useful in predicting tumor recurrence and early recurrence after surgery resection in patients with HCC confirming to Milan criteria.
Figures and Tables
Fig. 1
Treatment methods for recurrence tumor after initial hepatectomy. RFA, radiofrequency ablation; RR, repear resection; SLT, salvage liver transplantation; TACE, transcatheter arterial chemoembolization.

Fig. 2
ROC curve to assess the optimal cutoff value of NLR for tumor recurrence (1.505) (A) and early recurrence (1.945) (B). AUC, area under the curve; ROC, receiver operating characteristic; NLR, Neutrophil-to-Lymphocyte Ratio.

Fig. 3
(A and B) Kaplan-Meier survival analysis the overall survival and recurrence free survival of the present study patients. (C and D) Kaplan-Meier survival analysis patients with NLR >1.505 have a shorter recurrence free survival time, however, there were no difference on overall survival. NLR, Neutrophil-Lymphocyte Ratio.

Fig. 4
Kaplan-Meier survival analysis shows that the patients who experience early recurrence have a significantly lower overall survival.

Table 1
Comparison of Clinical and Pathological Characteristics with Normal and Elevated NLR

Table 2
Comparison of Clinical and Pathological Characteristics with Early and Late Recurrence

Table 3
Cox Proportional Hazards Model of Risk Factors for Recurrence Free Survival in 213 HCC Patients Confirming to Milan Criteria after Hepatectomy
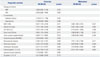
Table 4
Cox Proportional Hazards Model of Risk Factors for Overall Survival in 213 HCC Patients Confirming to Milan Criteria after Hepatectomy

Table 5
Cox Proportional Hazards Model of Risk Factors for Early Recurrence in 107 Recurrent Patients

References
1. Song IH, Kim KS. Current status of liver diseases in Korea: hepatocellular carcinoma. Korean J Hepatol. 2009; 15:15 Suppl 6. S50–S59.

2. Park KW, Park JW, Choi JI, Kim TH, Kim SH, Park HS, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008; 23:467–473.

3. Jung SH, Kim BH, Joung YH, Han YS, Lee BH, Dong SH, et al. [Clinical features of hepatocellular carcinoma in the 1990s]. Korean J Gastroenterol. 2003; 42:322–329.
4. Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003; 238:703–710.

5. Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001; 102:5–14.
6. Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008; 32:1757–1762.

7. Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013; 258:301–305.

8. Liao W, Zhang J, Zhu Q, Qin L, Yao W, Lei B, et al. Preoperative neutrophil-to-lymphocyte ratio as a new prognostic marker in hepatocellular carcinoma after curative resection. Transl Oncol. 2014; 7:248–255.

9. Huang ZL, Luo J, Chen MS, Li JQ, Shi M. Blood neutrophil-tolymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2011; 22:702–709.

10. Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012; 27:553–561.

11. Halazun KJ, Hardy MA, Rana AA, Woodland DC 4th, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009; 250:141–151.

12. Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013; 58:58–64.

13. Harimoto N, Shirabe K, Nakagawara H, Toshima T, Yamashita Y, Ikegami T, et al. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/lymphocyte ratio. Transplantation. 2013; 96:1008–1012.

14. Tajiri K, Kawai K, Minemura M, Yasumura S, Hosokawa A, Kawabe H, et al. Neutrophil/lymphocyte ratio as a prognostic indicator of hepatic arterial infusion chemotherapy with arterial cisplatin plus continuous 5-fluorouracil. Hepatol Res. 2015; 45:755–763.

15. Zheng YB, Zhao W, Liu B, Lu LG, He X, Huang JW, et al. The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac J Cancer Prev. 2013; 14:5527–5531.

16. Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994; 19:1513–1520.

17. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954; 7:462–503.

19. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–899.

20. Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013; 2013:187204.

21. Hotchkiss KA, Ashton AW, Klein RS, Lenzi ML, Zhu GH, Schwartz EL. Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Res. 2003; 63:527–533.
22. Marconi C, Bianchini F, Mannini A, Mugnai G, Ruggieri S, Calorini L. Tumoral and macrophage uPAR and MMP-9 contribute to the invasiveness of B16 murine melanoma cells. Clin Exp Metastasis. 2008; 25:225–231.

23. Peng SH, Deng H, Yang JF, Xie PP, Li C, Li H, et al. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol. 2005; 11:6521–6524.

24. Borish L, Rosenbaum R, Albury L, Clark S. Activation of neutrophils by recombinant interleukin 6. Cell Immunol. 1989; 121:280–289.

25. Maniecki MB, Etzerodt A, Ulhøi BP, Steiniche T, Borre M, Dyrskjøt L, et al. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int J Cancer. 2012; 131:2320–2331.

26. Varney ML, Olsen KJ, Mosley RL, Bucana CD, Talmadge JE, Singh RK. Monocyte/macrophage recruitment, activation and differentiation modulate interleukin-8 production: a paracrine role of tumor-associated macrophages in tumor angiogenesis. In Vivo. 2002; 16:471–477.
27. Zhao Q, Xiao X, Wu Y, Wei Y, Zhu LY, Zhou J, et al. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol. 2011; 41:2314–2322.

28. Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010; 51:154–164.

29. Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, Wang J, et al. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol. 2010; 185:1544–1549.

30. Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008; 15:1375–1382.

31. Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000; 89:500–507.

32. Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996; 83:1219–1222.

33. Cruz NG, Sousa LP, Sousa MO, Pietrani NT, Fernandes AP, Gomes KB. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2013; 99:85–92.

34. Shau WY, Shao YY, Yeh YC, Lin ZZ, Kuo R, Hsu CH, et al. Diabetes mellitus is associated with increased mortality in patients receiving curative therapy for hepatocellular carcinoma. Oncologist. 2012; 17:856–862.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download