Abstract
Purpose
Appropriate animal models of atherosclerotic plaque are crucial to investigating the pathophysiology of atherosclerosis, as well as for the evaluation of the efficacy and safety of vascular devices. We aimed to develop a novel animal model that would be suitable for the study of advanced atherosclerotic lesions in vivo.
Materials and Methods
Atherosclerotic plaque was induced in 24 iliac arteries from 12 rabbits by combining a high cholesterol diet, endothelial denudation, and injection into the vessel wall with either saline (n=5), olive oil (n=6), or inflammatory proteins [n=13, high-mobility group protein B1 (HMGB1) n=8 and tumor necrosis factor (TNF)-α n=5] using a Cricket™ Micro-infusion catheter. Optical coherence tomography (OCT) was performed to detect plaque characteristics after 4 weeks, and all tissues were harvested for histological evaluation.
Results
Advanced plaque was more frequently observed in the group injected with inflammatory proteins. Macrophage infiltration was present to a higher degree in the HMGB1 and TNF-α groups, compared to the oil or saline group (82.1±5.1% and 94.6±2.2% compared to 49.6±14.0% and 46.5±9.6%, p-value<0.001), using RAM11 antibody staining. On OCT, lipid rich plaques were more frequently detected in the inflammatory protein group [saline group: 2/5 (40%), oil group: 3/5 (50%), HMGB1 group: 6/8 (75%), and TNF-α group: 5/5 (100%)].
Acute coronary syndrome is well known to be caused by the rupture of vulnerable atheromatous plaques, resulting in insufficient perfusion of the myocardium.1 Human autopsy studies have indicated that vulnerable plaques are typically associated with a thin, inflamed fibrous cap overlaying a lipid core, increased neovascularization, medial and adventitial changes, intraplaque hemorrhage, inflammation, and positive vascular remodeling.234 In eroded plaques, which is another form of advanced plaque, thrombus occurs over the intima-deficient endothelium with thick fibroatheroma.24 The formation of vulnerable plaques and the development of sudden thrombus have been observed in postmortem humans with cardiovascular disease.3
Due to the lack of adequate animal models, pre-thrombotic pathophysiology and plaque vulnerability have been difficult to investigate in vivo. Several large animal models of vulnerable atherosclerosis and plaque rupture have been proposed.5678 Using a high cholesterol diet and balloon injury, followed by pharmacological triggering, a rabbit model of induced atherosclerotic plaque and atherothrombosis has been developed.68 However, these models show difficulties in developing consistent advanced atherosclerotic plaques, thereby limiting their usefulness to clinical researchers. To overcome these limitations, a reproducible porcine model of atherosclerosis, which utilized the direct injection of complex lipids into the vascular wall,9 was developed. Additionally, this approach resulted in focal lesions that were located in positively remodeled vessels that have increased neovascularization, as well as the release of pro-inflammatory chemokines.10
Atherosclerosis is a complex inflammatory and immune response disease, and plaque vulnerability is linked with increased levels of inflammation.11 As such, the direct injection of pro-inflammatory proteins into the vessel wall may trigger a more potent inflammatory response and consistently augment the atherosclerotic process within a short period of time. To date, no study has investigated the effects of injecting pro-inflammatory proteins directly into the vascular wall on inflammation and atherosclerosis. In this study, we selected the pro-inflammatory proteins high-mobility group protein B1 (HMGB1) and tumor necrosis factor (TNF)-α, because both of these chemokines are well known to promote the inflammatory process and mediate atherosclerosis. This study was designed to investigate the effect of injecting pro-inflammatory proteins into the vessel wall on the development of advanced plaques and inflammation.
A total of 16 male New Zealand white rabbits (12 months old, weighing 3.0–4.0 kg) were used in the study. Four rabbits did not complete the protocol. Finally, 12 rabbits were included in this study. The experimental protocol is shown in Fig. 1.
All rabbits were given a high cholesterol diet (2% cholesterol, Scientific Animal Food & Engineering, Augy, France) 1 week prior to and 4 weeks after the balloon injury and intramuscular injection. After premedication with antibiotics and analgesics, anesthesia was induced by intramuscular injection with an appropriate mixture of tiletamine–zolazepam (10 mg/kg, Zoletil®, Virvac, Fort Worth, TX, USA) and xylazine (0.5 mg/kg , Rompun®, Bayer, Leverkusen, Germany), then maintained with 1–2% of isoflurane (Forane®, JW Pharm, Seoul, Korea) and oxygen. Access to the iliac artery was obtained via the carotid artery, using a sterile surgical technique. Heparin (150 units/kg) was injected to maintain an activated clotting time >250 s before catheterization. Based on quantitative angiography (QA), an oversized balloon inflation with a 1.3:1.0 (balloon:artery ratio) was applied two times for 30 seconds within both iliac arteries using a 15-mm-length balloon. Following balloon injury, oil, inflammatory proteins+oil, or saline was delivered to each iliac artery using a Cricket™ Micro-Infusion catheter (Mercator Medsystems, San Leandro, CA, USA) over a guidewire under fluoroscopic guidance. Following the compound delivery, angiography and OCT examination were performed. All animals received 40 mg aspirin (~10 mg/kg) and clopidogrel (75 mg/daily) after the procedure. The animals were euthanized 4-weeks post-procedure, and the iliac arteries were harvested.
The study protocol was approved by the local institutional animal care and use committee (Medi Kinetics, MK-IACUC: 111027-001 and Cardiovascular Production Evaluation Center, Yonsei University College of Medicine). All animals received humane care in compliance with the Animal Welfare Act and the "Principles of Laboratory Animal Care" formulated by the Institute of Laboratory Animal Resources (National Research Council, NIH Publication No. 85-23, revised 1996).
A total of 32 iliac arteries from 16 rabbits were randomly assigned into four groups: saline injection group (Daihan Pharm Co., Seoul, Korea, n=8), olive oil injection group (Sigma Aldrich, St. Louis, MO, USA, n=8), HMGB1 (A&RT, Daejeon, Korea)+oil injection (n=8), and the TNF-α (Prospec, Ness-Ziona, Israel)+oil group (n=8). The operator was blinded to each artery material that was randomly selected and injected differently to both sides of iliac arteries. Immediately after the balloon injury, endovascular intramural injection using a Cricket™ Micro-Infusion catheter was performed. The infusion catheter is designed to deliver the material to the adventitia of the vessel wall via needle injection. The Cricket™ Micro-Infusion catheter was placed within the balloon-injured site, and the balloon was inflated. The vessel wall was penetrated by the needle. Complete occlusion after balloon inflation and shape of needle were confirmed by angiography. The infusion catheter system delivered 200 µL of saline, oil, HMGB1, or TNF-α (inflammatory proteins, 20 ug/200 µL dilution into saline) into the vessel wall. Each animal received two treatments within each iliac artery. The circumferential injection of solutions was repeated four times within the injured vessel segment to achieve a total of 1 cc of the target dose. Angiography and optical coherence tomography (OCT) were performed twice: immediately post-procedure and 4-weeks post-procedure. After the autopsy, 15 mm of vessel was dissected into three equal parts (5 mm each) and stored.
Plasma levels of total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) were measured by DRI-CHEM 4000i (Fujifilm, Tokyo, Japan) (TCHO-P, TG-P, HDL-C-PD) in blood samples before, 1 week, and 5 weeks after the introduction of the high cholesterol diet. Blood samples were collected from the carotid arteries of the rabbits.
Under anesthesia, all animals were euthanized immediately after the follow-up imaging was complete. Iliac vessels (5 mm in length) were fixed by continued perfusion with 10% normal buffered formalin. To assure the proper identification, right iliac arteries were marked at the distal end with a 4-0 silk suture, and then both arteries were carefully cut and immersion-fixed overnight before the histological processing. For paraffin embedding, 4 mm in length of vessel was placed intact into a single cassette, and processed through a graded series of alcohols and xylenes. After processing, the specimen was embedded as a single paraffin block. Paraffin sections were cut on a microtome at 4 microns, mounted on microscope slides (Superfrost Plus, Fisher Scientific, Waltham, MA, USA), and stained with hematoxylin and eosin (H&E) staining, Masson's trichrome staining, Movat's pentachrome staining, and Sirius red stain for collagens. Frozen sections were cut on a microtome at 10 microns, and mounted a 1 mm length of vessel for Oil red O lipid staining.
In addition, the induction of inflammation and the expression of HMGB1 and TNF-α were evaluated in arterial samples using a mouse monoclonal anti-rabbit macrophage antibody (RAM11, DAKO, Santa Clara, CA, USA), the anti-body for HMGB1 (NOVUS, Littleton, CO, USA), TNF-α (ABCAM, Cambridge, MA, USA), and the receptor for advanced glycation end products (RAGE, LSBio, Seattle, WA, USA). Changes in smooth muscle acting (SMA) were also evaluated with the anti-SMA (ABCAM Cambridge, MA, USA), using a general immunohistochemistry (IHC) protocol (Supplementary Method 1, only online).
Atherosclerotic plaques types were classified into early (type II, fatty streak, and type III, pre-atheroma) and advanced (type IV, atheroma; type V, fibroatheroma) plaques according to the American Heart Association (AHA) criteria.12 All pathologic slides were reviewed by one pathologist (S.H.K.) to determine the histomorphometric analysis and AHA classification.
The cross-sectional areas [external elastic lamina (EEL), internal elastic lamina (IEL) stenosis and lumen] were measured on stained slides using digital morphological analysis. Digital images of the vessels were scanned using a Leica SCN400 (Wetzlar, Germany) and histomorphometry was performed using Leica Application Suite (LAS) 4.2 (Leica, Wetzlar, Germany).
1) Media=EEL-IEL
2) Intima area=IEL-Lumen
3) Plaque area=media+intima area
Total RNA was isolated from the iliac vessels (5 mm length) using a tissue grinder motor (Bel-Art Products, Wayne, NJ, USA) and QIAzol lysis reagent (QIAGEN, Hilden, Germany). The concentration of RNA was measured by Nanodrop 2000/2000c (Thermo Scientific, Long Beach, NY, USA). Complementary DNA (cDNA) was synthesized using Quantitect Reverse Transcription (QIAGEN) and PCR using the AccuPower PCR Premix (Bioneer, Daejeon, Korea). All PCR products were separated by electrophoresis on 2% agarose gels diluted in TAE. Sizes were compared to an included 100 bp DNA ladder (DYNE Bio, Seongnam, Korea), and PCR products were visualized using Loading STAR (DYNE Bio, Seongnam, Korea).
The rabbit gene primer sequences used for PCR were: RAGE, forward 5H-ATGGTCACCCTCAGATCA-3' and reverse 5'-CT GAAGAGAACCTGGGAG-3'; HMGB1, forward 5'-GTTCTGAG TATCGCCCCAAA-3' and reverse 5'-TCCTCCTCATCCTCTTC GTC-3'; TNF-α, forward 5'-ATGGTCACCCTCAGATCA-3' and reverse 5'-CTGAAGAGAACCTGGGAG-3'; GAPDH, forward 5'-AGGTCATCCACGACCACTTC-3' and reverse 5'-GTGAGTT TCCCGTTCAGCTC-3'. Relative mRNA levels were determined by comparison to the GAPDH mRNA.
Iliac vessels (5 mm length) were homogenized using Tissue Grinder Motor (Bel-Art Products, Wayne, NJ, USA) and lysed with RIPA buffer (Biosesang, Seongnam, Korea) containing a cOmplete Mini, ethylenediaminetetraacetic acid (EDTA)-free protease inhibitor cocktail® (Roche, Basel, Switzerland). The protein concentrations were measured by bicinchoninic acid protein assay. Iliac artery lysates were loaded and separated on 12% SDS-PAGE gels and transferred onto Immuno-Blot® PVDF Membrane (BIO-RAD, Hercules, CA, USA). Membranes were blocked by a 5% skim milk (Noble Bio, Hwaseong, Korea) dilution in Tris-Buffered Saline and Tween 20 (TBS-T) at room temperature for 1 hour, and were subsequently washed three times in TBS-T. Membranes were incubated in TBS-T with primary antibodies against HMGB1, TNF-α, and RAGE (all 1:1000 in 5% BSA, Biosesang, Seongnam, Korea) overnight at 4℃. Membranes were washed three times in TBS-T and incubated with horseradish peroxidase conjugated secondary antibody at room temperature for 1 hour. All blots were probed using GAPDH as a loading control. Densitometry analysis was performed with Image J software (National Institutes of Health, Bethesda, MD, USA).
Quantitative coronary angiography analysis was performed immediately before the procedure and 4-weeks post-procedure using an off-line quantitative coronary angiographic system (CAAS System; Pie Medical Instruments, Maastricht, the Netherlands) in an independent core laboratory (Cardiovascular Research Center, Seoul, Korea). Using the guiding catheter for magnification-calibration, the reference vessel diameter (RD) and minimum luminal diameter (MLD) were measured from diastolic frames in a single, matched view showing the smallest MLD. Percent diameter of stenosis was calculated according to the following formula: percent diameter stenosis=[(mean RD-MLD)/mean RD]×100, mean RD=(proximal RD+distal vessel diameter)/2.
OCT was performed using the C7-XR imaging systems (Light-Lab Imaging, Inc., St. Jude Medical, St. Paul, MN, USA). The OCT catheter was pulled back at 20 mm/s, and OCT images were generated at 100 frames/s. Contrast media were continuously flushed through a guiding catheter at a rate of 4–5 mL/s for 3–4 seconds. Images were continuously acquired and stored digitally for subsequent analyses. All OCT images were analyzed at a core laboratory (Cardiovascular Research Center, Seoul, Korea) by analysts who were blinded to procedural information. The histological images were compared to their corresponding OCT pullback lesion segments until the best and closest visual match was found (Fig. 2). This match was performed with the consideration to the anatomical features (luminal shape, neointimal thickness, presence of neovascularization, etc.), the proximity to side branches, and the length from the balloon injury edge to the location of the histology samples (provided by the histological laboratory). Plaques were classified as 1) fibrous (homogeneous, high backscattering region) or 2) lipid (low-signal region with diffuse border).13 Macrophage infiltration within the lesion was characterized by increased signal intensity within the lesion accompanied by the heterogeneous backward shadows.14
Statistical analyses were performed using SPSS v20.0 (SPSS Inc., Chicago, IL, USA). All data are expressed as mean±SEM. Continuous variables were compared using t-test (comparison of two groups) and ANOVA (comparison of 3 or more groups). If data distributions were skewed, a non-parametric test was used for comparison. A p-value of<0.05 was considered statistically significant.
Two animals died immediately post-procedure due to cardiac arrhythmia, and two others of saline group 1 site and TNF-α group 3 sites died due to liver failure before evaluation of lesions within 5 weeks. These could have stemmed from complications resulting from the high cholesterol diet. Thus, 24 target lesions within 24 iliac arteries were harvested from 12 rabbits after 5 weeks [saline (n=5), oil (n=6), HMGB1 (n=8), and TNF-α (n=5)].
The mean TC level for all animals was 26.4±7.0 mg/dL at baseline. This significantly increased to 874±63 mg/dL 5 weeks after the initiation of the 2% cholesterol diet (1 week before the procedure and 4 weeks after the procedure, p<0.001). Low-density lipoprotein cholesterol, TG, and HDL-C were 743±60 mg/dL, 182±32 mg/dL, and 96±6 mg/dL, respectively, at 5 weeks after the cholesterol diet was initiated.
Five weeks after the initiation of the high cholesterol diet, minimal lumen diameter was similar in all groups (saline group: 0.89±0.09 mm, oil group: 0.93±0.07 mm, HMGB1 group: 0.90±0.10 mm, and TNF-α group: 1.01±0.08 mm, p-value by ANOVA= 0.546) (Table 1). The percentage of diameter stenosis was also not significantly different among all groups (saline group: 35.82±2.35%, oil group: 36.93±4.23%, HMGB1 group: 39.73±3.13%, and TNF-α group: 34.55±2.74%, p-value by ANOVA=0.813) (Table 1).
Atherosclerotic plaques developed in all the injected segments. A representative histologic image is shown in Fig. 2. The advanced plaque type (III, VI, and V by AHA criteria) was more frequently observed in the pro-inflammatory protein injected group, compared to all other groups (Fig. 2). Plaque area tended to be higher in oil, HMGB+oil, and TNF-α+oil groups, compared to the saline group (saline group: 1.22±0.17 mm2, oil group: 1.64±0.33 mm2, HMGB1 group: 1.62±0.19 mm2, and TNF-α group: 1.55±0.26 mm2, p-value by ANOVA=0.696) (Table 1).
IHC demonstrated that percent area stained positive for RAGE, HMGB1, and TNF-α tended to be higher in the oil or pro-inflammatory proteins group, compared with the saline group. Macrophage infiltration within the plaques was significantly higher in the HMGB1 and TNF-α groups, compared to both the oil and the saline groups (82.1±5.1% and 94.6±2.2% compared to 49.6±14.0% and 46.5±9.6%, p-value by ANOVA=0.014) (Fig. 3). RT-PCR showed that the expression of RAGE mRNA was significantly higher in the HMGB1 group, compared to the saline group, and tended to be higher in the TNF-α group than the saline group (p=0.054). The TNF-α group had a significantly higher expression of HMGB1 and TNF-α mRNA expression, compared to the saline and oil groups (Fig. 4). RAGE protein expression was significantly higher in the HMGB1 group than the saline, oil, and TNF-α groups, as assessed by the western blot (Fig. 5).
Figs. 6 and 7 displays representative histological and OCT images for comparison. The 24 histologic lesions included in this study yielded a total of 24 OCT histology co-registrations. The lumen area, plaque area, and percent area stenosis were not significantly different among the four groups. However, the plaque area tended to increase in the HMGB1 and TNF-α groups (saline group: 1.88±0.24 mm2, oil group: 2.34±0.17 mm2, HMGB1 group: 2.71±0.29 mm2, and TNF-α group: 3.27±0.29 mm2, p-value by ANOVA=0.065).
By qualitative analysis, all the lipid rich plaques assessed by OCT were well-matched with histologic findings. The proportion of plaques found to be lipid rich was different among the groups. Fig. 6E demonstrates the breakdown of plaque classification by group [saline group: 2/5 (40%), oil group: 3/5 (50%), HMGB1 group: 6/8 (75%), and TNF-α group: 5/5 (100%)]. Plaques determined to be class III–V were classified as lipid rich.
There are two key findings from this study. First, our data suggest that the induction of inflammation via direct injection of pro-inflammatory proteins, such as HMGB1 or TNF-α, into the iliac artery increases macrophage infiltration and accelerates inflammation within the vessel wall. Second, advanced atheromatous plaques, including lipid rich plaques, were more frequently induced by the injection of pro-inflammatory proteins, compared to the controls. The current study may provide a useful in vivo modality for developing advanced atheromatous plaques by direct injection of pro-inflammatory proteins.
A rabbit model of atherosclerosis has been proposed to easily induce foam cell-rich plaques, because of its unique sensitivity to hypercholesterolemia.6 The iliac artery or aorta of the rabbit is frequently used as a model for preclinical cardiovascular research studies due to its similar morphology with the coronary artery of humans.8 However, its use is limited by inter-individual variations in plasma cholesterol levels and lesion characteristics in response to a cholesterol diet. Furthermore, this model has a risk of lipid toxicity resulting from a prolonged exposure to a high cholesterol diet.6 In addition, balloon injury followed by a high cholesterol diet usually results in the induction of early stage atherosclerotic plaques rather than advanced plaques. In this study, in order to overcome this variability of developed atheromatous plaque, we modified the existing protocols in animal models. Previously, Granada, et al.9 proposed a method for inducing atherosclerotic plaques by combining balloon injury and the vascular wall injection of lipidic substances in a swine model. In their study, atherosclerotic lesions containing inflammatory cells, positive remodeling, and neovascularization all developed and resembled the early stages of human atherosclerosis.5 Furthermore, the intramural injection of complex lipids into the coronary arteries induced an increased expression of vascular endothelial grow factor (VEGF) and monocyte chemoattractant protein 1 (MCP-1).10
However, to the best of our knowledge, no study has investigated the induction of an atherosclerotic plaque in a rabbit model by the direct injection of a lipidic substance or pro-inflammatory proteins into the vessel wall. We observed the developed plaque area to be similar among the groups injected with either saline, lipid, or lipid+pro-inflammatory protein. However, macrophage accumulation was more prominent in the pro-inflammatory injected groups. Furthermore, the proinflammatory groups had an increased expression of TNF-α and HMGB1 within the intima and media of the vessel, compared with either the saline or oil injected groups. This finding suggests that direct tissue injection of pro-inflammatory proteins may induce potent inflammation and promote the development of an advanced atheromatous plaque.
We applied two pro-inflammatory proteins in this study, TNF-α and HMGB1. TNF-α is a well-known potent pro-inflammatory cytokine involved in the pathogenesis of arteriosclerosis. It promotes the inflammatory response, which causes many of the clinical problems associated with autoimmune disorders, such as inflammatory disease.1115 HMGB1 is a potent proinflammatory protein ligand that mediates inflammation by activating innate immunity receptors, such as toll-like receptor (TLR) and RAGE.16 HMGB1 passively diffuses out of necrotic or apoptotic cells. It works as a pro-inflammatory mediator that activates immune competent cells, which in turn augment the inflammatory responses through the discharge of pro-inflammatory cytokines.17 Studies have shown that HMGB1 is highly expressed in the human carotid endarterectomy specimens.18 In this study, injection of HMGB1 into the vessel wall was associated with increased expression of RAGE and with increased recruitment of macrophages to the lesion. These data suggest that the HMBG1 injection may induce an upregulation of RAGE expression and activation, resulting in an increased recruitment of macrophages through the expression of cytokines and cell adhesion molecules.16
OCT is a well-established, accurate imaging modality with a high resolution, capable of evaluating the characteristics of atheromatous plaques in clinical situations.1316,19 Here, using OCT, we were able to show that HMGB1 and TNF-α groups had a higher incidence of lipid rich plaques, compared to both the oil or saline groups. This finding confirmed histological studies that showed HMGB1 and TNF-α groups have more advanced plaques, compared to the saline and oil groups. OCT indicated that the diameter and area of the stenosis were not different between the four groups.
The main limitation of this study was its short duration, which did not allow for the development of an established necrotic core, intra-plaque hemorrhage, or calcified plaques. Furthermore, plaque rupture or thrombus, which is the main pathogenic mechanism of acute coronary syndrome, was not observed in this experimental model. However, these limitations do not detract from the central merit of this work, which shows that a consistent model of advanced atherosclerosis has been developed. Therefore, our study seems as a cost-effective and short-time method that induces atherosclerotic lesions in rabbit. The lack of adequate medium to large sized animal models for atherosclerosis has been a major limitation for animal research in the cardiovascular field. We believe this model may be useful for further study investigating the pathogenesis and treatment of atherosclerosis. Also, it could be useful for evaluations of device or screening therapeutic substances for treatment cardiovascular disease in the atherosclerosis research field. Another limitation of our study is that we could not compare the vascular remodeling process after injection, because we did not measure vascular structure before injection in our experiment. However, we can assume that there may be some positive remodeling process in inflammatory proteininjected group, considering increased in plaque volume in these groups.
In conclusion, direct injection of inflammatory proteins into the iliac artery wall significantly increased macrophage infiltration, and induced the formation of a lipid rich plaque. Our data indicate that the rabbit model developed here may be useful for in vivo studies investigating atherosclerosis.
Figures and Tables
 | Fig. 1Experimental protocol. Schematic view of study timeline. Upon arrival to the animal facility, rabbits were started on a daily high cholesterol diet for 5 weeks. One week later, balloon injury was induced, and inflammatory proteins were injected. Body weight and serums were assessed before the high cholesterol diet and before the sacrifice at 5 weeks. OCT was assessed at the beginning and end of the study. Animals were euthanized 4 weeks after the injection procedure, and iliac arteries were harvested at that time. OCT, optical coherence tomography. |
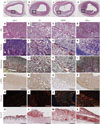 | Fig. 2Morphologic changes of rabbit iliac artery analysis. Tissue staining of induced atherosclerosis iliac artery plaques in four groups of rabbits. The lipid content of the plaques is indicated by Oil red O staining; the SMA content of the plaques is detected by immunohistochemical staining of α-SMA; the collagen content of the plaques is represented by Sirius red staining visualized under polarized light. Representative examples of H&E [a-d (amplification ×30), A-D], Masson's trichrome (E-H), Masson's pentachrome (I-L), α-SMA (M-P), Sirius red (Q-T), Oil red O (U: saline injection plaque, V: oil injection plaque, W: HMGB1 injection plaque, X: TNF-α injection plaque) in the rabbit iliac (amplification ×100). a: Saline injection plaque A, E, I, M, Q: higher magnification taken from the black box in a. b: Oil injection plaque B, F, J, N, R: higher magnification taken from the black box in b. c: HMGB1 injection plaque C, G, K, O, S: higher magnification taken from the black box in c. d: TNF-α injection plaque D, H, L, P, T: higher magnification taken from the black box in d (scale bars=100 µm). SMA, smooth muscle actin; HMGB1, high-mobility group protein B1; TNF-α, tumor necrosis factor-α; H&E, hematoxylin and eosin. |
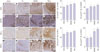 | Fig. 3Morphologic changes of rabbit iliac artery analysis according to immunohistochemistry. Tissue staining of induced atherosclerosis iliac artery plaques in four groups of rabbits. The inflammation content of the plaques is detected by immunohistochemical staining for RAGE, HMGB1, and TNF-α; the macrophage content of the plaques is detected by immunohistochemical staining for the anti-rabbit macrophage clone RAM11. Representative examples of immunohistochemical stain of RAGE (A-D), HMGB1 (E-H), TNF-α (I-L), and RAM11 (M-P) in the rabbit iliac (amplification ×100) (scale bars=100 µm). Lesions of macrophage were markedly less for saline group and oil group, which showed significant differences in the total percent area in comparison to the HMGB1 group and TNF-α group on RAM11 immunohistochemical staining. *p<0.05, compared with the Saline group, †p<0.05, compared with the Oil group. RAGE, receptor for advanced glycation end products; HMGB1, high-mobility group protein B1; TNF-α, tumor necrosis factor-α. |
 | Fig. 4The relative mRNA levels in induced atherosclerosis of rabbit iliac artery. Reverse transcription (RT)-PCR analysis of RAGE, HMGB1, and TNF-α mRNA expression in iliac arteries from four groups of rabbits A. Representative data showing the mRNA expressions of RAGE, HMGB1, and TNF-α in iliac arteries from four groups of rabbits (normalized with GAPDH as the house keeping gene) B, C, and D. The data in the bar graph are quantified ratios of the signal for RAGE, HMGB1, and TNF-α to that for GAPDH set at fold increase. Data are presented as the mean±SEM. *p<0.05, compared with the Saline group, †p<0.05, compared with the Oil group. RAGE, receptor for advanced glycation end products; HMGB1, high-mobility group protein B1; TNF-α, tumor necrosis factor-α; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SEM, standard error of the mean. |
 | Fig. 5The relative protein levels in induced atherosclerosis of rabbit iliac artery. Western blot analysis of RAGE, HMGB1 and TNF-α mRNA expressions in iliac artery from four groups of rabbits A. Representative data showing the protein expressions of RAGE, HMGB1 and TNF-α levels in iliac arteries from four groups of rabbits (normalized with GAPDH as the house keeping gene) B, C, and D. The data in the bar graph are quantified ratios of the signal for RAGE, HMGB1, and TNF-α to that for GAPDH set at fold increase. Data were presented as the mean±SEM. *p<0.05, compared with the Saline group, †p<0.05, compared with the Oil group, ‡p<0.05, compared with the TNF-α group. RAGE, receptor for advanced glycation end products; HMGB1, high-mobility group protein B1; TNF-α, tumor necrosis factor-α; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SEM, standard error of the mean. |
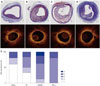 | Fig. 6OCT images of matched rabbit iliac artery histologic sections and histologic classification based on AHA criteria. OCT images obtained in vivo were matched with histological sections. Saline group A; Oil group B; HMGB1 group C; TNF-α group D. Histologic classification by AHA criteria E. Plaque characteristics were categorized into early (type II, fatty streak, and type III, pre-atheroma) and advanced (type IV, atheroma; type Va, fibroatheroma; type Vc, fibrotic; and type VI, complicated). AHA, American Heart Association; OCT, optical coherence tomography; HMGB1, high-mobility group protein B1; TNF-α, tumor necrosis factor-α. |
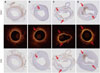 | Fig. 7OCT matching with histomorphometry for macrophage infiltration. OCT images obtained in vivo were matched with histomorphological section of rabbit models for macrophage infiltration. Tissue staining of induced atherosclerosis iliac artery plaques in 4 groups of rabbits. The macrophage content of the plaques is detected by immunohistochemical staining for the anti-rabbit macrophage clone RAM11 and CD68 (arrows). Saline group A; Oil group B; HMGB1 group C; TNF-α group D. OCT, optical coherence tomography; HMGB1, high-mobility group protein B1; TNF-α, tumor necrosis factor-α. |
Table 1
Morphological Parameters in QA, OCT, and Histology

QA, quantitative angiography; RD, reference diameter; MLD, minimal luminal diameter; DS, diameter stenosis; I/P, intimal plaque ratio; OCT, optical coherence tomography; HMGB1, high-mobility group protein B1; TNF-α, tumor necrosis factor-α.
Values are n (%) or mean±SEM.
*p<0.05, compared with the Saline group.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2012 (6-2012-0131), by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2055584), by a grant from the Korea Healthcare Technology R&D Project, Severance Integrative Research Institute for Cerebral & Cardiovascular Diseases, Ministry for Health & Welfare Affairs, Republic of Korea (HI08C2149) and the Cardiovascular Research Center, Seoul, Korea.
References
1. Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012; 125:1147–1156.

2. Otsuka F, Joner M, Prati F, Virmani R, Narula J. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014; 11:379–389.

3. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006; 47:8 Suppl. C13–C18.

4. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014; 114:1852–1866.

5. Granada JF, Wallace-Bradley D, Win HK, Alviar CL, Builes A, Lev EI, et al. In vivo plaque characterization using intravascular ultrasound-virtual histology in a porcine model of complex coronary lesions. Arterioscler Thromb Vasc Biol. 2007; 27:387–393.

6. Phinikaridou A, Hallock KJ, Qiao Y, Hamilton JA. A robust rabbit model of human atherosclerosis and atherothrombosis. J Lipid Res. 2009; 50:787–797.

7. Granada JF, Kaluza GL, Wilensky RL, Biedermann BC, Schwartz RS, Falk E. Porcine models of coronary atherosclerosis and vulnerable plaque for imaging and interventional research. EuroIntervention. 2009; 5:140–148.

8. Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, et al. Rabbit models for the study of human atherosclerosis: from pathophysiological mechanisms to translational medicine. Pharmacol Ther. 2015; 146:104–119.

9. Granada JF, Moreno PR, Burke AP, Schulz DG, Raizner AE, Kaluza GL. Endovascular needle injection of cholesteryl linoleate into the arterial wall produces complex vascular lesions identifiable by intravascular ultrasound: early development in a porcine model of vulnerable plaque. Coron Artery Dis. 2005; 16:217–224.

10. Tellez A, Schuster DS, Alviar C, López-Berenstein G, Sanguino A, Ballantyne C, et al. Intramural coronary lipid injection induces atheromatous lesions expressing proinflammatory chemokines: implications for the development of a porcine model of atherosclerosis. Cardiovasc Revasc Med. 2011; 12:304–311.

11. Ridker PM, Lüscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014; 35:1782–1791.

12. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995; 15:1512–1531.

13. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012; 59:1058–1072.
14. Tearney GJ. OCT imaging of macrophages: a bright spot in the study of inflammation in human atherosclerosis. JACC Cardiovasc Imaging. 2015; 8:73–75.
15. McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009; 6:410–417.

16. Park S, Yoon SJ, Tae HJ, Shim CY. RAGE and cardiovascular disease. Front Biosci (Landmark Ed). 2011; 16:486–497.

17. Hirata Y, Kurobe H, Higashida M, Fukuda D, Shimabukuro M, Tanaka K, et al. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis. 2013; 231:227–233.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download