Abstract
Purpose
To investigate associations between dyspnea and clinical outcomes in patients with non-small cell lung cancer (NSCLC).
Materials and Methods
From 2001 to 2014, we retrospectively reviewed the prospective lung cancer database of St. Paul's Hospital at the Catholic University of Korea. We enrolled patients with NSCLC and evaluated symptoms of dyspnea using modified Medical Research Council (mMRC) scores. Also, we estimated pulmonary functions and analyzed survival data.
Results
In total, 457 NSCLC patients were enrolled, and 259 (56.7%) had dyspnea. Among those with dyspnea and whose mMRC scores were available (109 patients had no mMRC score), 85 (56.6%) patients had an mMRC score <2, while 65 (43.3%) had an mMRC score ≥2. Significant decreased pulmonary functions were observed in patients with dyspnea. In multivariate analysis, aging, poor performance status, advanced stage, low forced expiratory volume in 1 second (%), and an mMRC score ≥2 were found to be significant prognostic factors for patient survival.
Lung cancer is a commonly diagnosed cancer, and the leading cause of cancer death around the world.1 The socioeconomic burden of lung cancer in many countries has increased drastically. According to a survey by the European Union, lung cancer had the highest economic cost (€18.8 billion, 15% of overall cancer costs) among all cancers in 2009.2 Advances in treatment modalities (e.g., surgery, radiation, chemotherapy, and molecular targeted therapy) have been made, and have improved patient outcomes over the past few decades. Additionally, as a screening tool for lung cancer, low dose computed tomography has been shown to reduce the mortality of patients with lung cancer by up to 20%, compared with conventional radiography.3 However, the mortality rate of lung cancer still remains high, and causes tremendous physical and emotional distress to patients.45
To develop more effective and individualized treatment for patients with lung cancer, many investigations on prognostic factors have been conducted. As a result, several clinical factors, including aging, male sex, poor performance status, advanced stage disease, and smoking, have been found to be associated with poor prognosis.6 Most lung cancer patients have smoking history and accompanying chronic obstructive pulmonary disease (COPD).7 COPD is a chronic progressive inflammatory airway disease that primarily occurs in smokers. COPD increases the risk of lung cancer, even after controlling for other important variables, and it is also closely related to poor clinical outcomes.8
Dyspnea is one of the most common symptoms in patients with lung cancer, and clinicians encounter it frequently at initial presentation. Moreover, with aggressive or conservative management of lung cancer, most patients with advanced lung cancer usually suffer from dyspnea. The degree of dyspnea is an important and validated factor for assessment of quality of life (QOL) in cancer patients.910 In addition, improvement of health-related QOL and symptoms, such as dyspnea, are related with the efficacy of chemotherapeutic regimens and favorable outcome in lung cancer.11 Therefore, clinicians should be concerned with their patients' dyspnea for improving clinical outcomes. However, the prognostic role of dyspnea in patients with lung cancer has not been studied well.
In the present study, we investigated the association between the presence or degree of dyspnea and clinical outcomes to identify the prognostic role of dyspnea in patients with non-small cell lung cancer (NSCLC).
We retrospectively reviewed the lung cancer database of St. Paul's Hospital at the Catholic University of Korea. From 2001 to 2014, we recruited patients who were diagnosed with lung cancer histologically and/or cytologically into our lung cancer registry. Following inclusion, clinical data, questionnaire, pulmonary function, and clinical outcomes from each patient were recorded prospectively. In this study, we enrolled patients who were diagnosed with NSCLC and had clinicopathological information on age, sex, smoking history, histologic type, stage, and Eastern Cooperative Oncology Group (ECOG) performance status in the lung cancer database.
We defined a current smoker as a patient who continued smoking upon diagnosis or stopped smoking less than 1 month before diagnosis of lung cancer. A former smoker was defined as a patient who had stopped smoking at least 1 month before the diagnosis. Patients who had never smoked or had smoked fewer than 100 cigarettes in their lifetime were defined as a never smoker. Histologic types were divided into adenocarcinoma, squamous cell carcinoma, large cell carcinoma, adenosquamous cell carcinoma, adenocarcinoma in situ, and other lung cancer. TNM stage was classified according to the 7th American Joint Committee on Cancer tumor, node, and metastasis classification.
At the time of diagnosis, we evaluated symptoms of dyspnea using questionnaires, and assessed pulmonary function parameters in each patient. Patients were categorized into two groups according to the presence of dyspnea at initial presentation. In patients with dyspnea, the degree of dyspnea was measured by the modified Medical Research Council (mMRC) dyspnea scale.12 The mMRC dyspnea scale can range from 0 to 4 (Grade 0, breathless with strenuous exercise; Grade 1, short of breath when hurrying on level ground or walking up a slight hill; Grade 2, walks slower than people of the same age on a level plane because of breathlessness; Grade 3, stops for breath after walking about 100 yards; Grade 4, too breathless to leave the house or becomes breathless when dressing or undressing). Pulmonary function parameters, such as forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), forced expiratory ratio (FEV1/FVC), forced expiratory flow 25–75% (FEF25–75), diffusion capacity of the lung for carbon monoxide (DLCO), and residual volume (RV), were collected. Using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, an obstructive pattern was defined as a FEV1/FVC ratio less than 0.7. In addition, patients were categorized into four classes according to their airflow limitation severity: GOLD1 (mild) was FEV1 ≥80% predicted; GOLD2 (moderate) was 50%≤FEV1<80% predicted; GOLD3 (severe) was 30%≤ FEV1<50% predicted; and GOLD4 (very severe) was FEV1 <30% predicted.13
We searched for survival data from the lung cancer database of St. Paul's Hospital. The last follow-up date was July 2014. This study was approved by the Institutional Review Board/Ethics Committee of St. Paul's Hospital, the Catholic University of Korea (PC15RISI0053).
Frequencies and proportions were used to present categorical clinical variables. To estimate the association between dyspnea and other categorical clinical variables, we used the Pearson's χ2 tests. The unpaired t-test was used to compare pulmonary function parameters between two groups according to the presence of dyspnea. Survival curves according to the putative prognostic factors were drawn using the Kaplan-Meier method, and survival differences were analyzed by the log-rank test. The two-sided significance level was set at p<0.05. The factors that were significantly associated with patient survival in univariate analysis, were further evaluated in multivariate analysis. The Cox proportional hazards modeling technique was applied to identify independent prognostic factors. All statistical analyses were performed using SPSS statistical software, version 20.0 (IBM SPSS statistics, version 20.0 for Windows; SPSS Inc., Chicago, IL, USA).
Four hundred and fifty-seven patients were enrolled in this study. The median age thereof was 68.5 years. Symptoms of dyspnea were detected in 259 (56.7%) patients at initial presentation. The existence of dyspnea was significantly associated with smoking status and ECOG performance status. However, it was not associated with age, sex, and histologic type or stage (Table 1).
Among 259 patients who complained of dyspnea at initial presentation, we were able to obtain mMRC scores for 150 (58%) patients. mMRC score showed the following distribution: 23 patients (15.3%) with mMRC 0, 62 (41.3%) with mMRC 1, 23 (15.3%) with mMRC 2, 27 (18.0%) with mMRC 3, and 15 (10.0%) with mMRC 4 (Fig. 1).
We compared pulmonary function parameters according to the presence of dyspnea. All spirometric values, including FVC (%), FEV1 (%), FEV1/FVC (%), FEF25–75 (%), and DLCO (%), were significantly lower in patients with dyspnea than those without dyspnea (Table 2). However, there was no significant difference in the level of RV between the two groups.
According to the results from initial data analysis, there was a substantial amount of missing data on mMRC. All of the missing values were imputed by linear regression model.14 Using this model, we acquired a new data set that consisted of the original data and the imputed values. The R-squared of this model was 0.5596.
Among the 246 patients who had dyspnea symptoms and spirometric values, 145 patients (56%) had an obstructive pattern (FEV1/FVC <0.7). These patients were divided into four classes according to FEV1 (%) value using the GOLD criteria. Forty one patients (28%) were classified as GOLD1 (mild COPD), 76 (52%) as GOLD2 (moderate COPD), 26 (18%) as GOLD3 (severe COPD), and two (1%) as GOLD4 (very severe COPD) (Fig. 2).
The overall median survival for all patients was 10.3 months. The overall survival of patients with dyspnea was significantly lower than that for patients without dyspnea (median survival, 7.9 months vs. 15.3 months, p<0.001). A significant difference in median survival was also found between patients with mMRC grade 0 or 1 and those with mMRC grade 2 or higher (12.3 months vs. 5.8 months, p<0.001) (Fig. 3). In univariate analysis, presence of dyspnea and the spirometric values FEV1/FVC (%) and FEV1 (%) were significantly associated with patient survival in addition to other prognostic factors, including aging, poor performance status, smoking history, and advanced stage. Subsequently, we performed multivariate analysis to identify independent prognostic factors of patients with lung cancer. Age [hazard ratio (HR), 1.60; 95% confidence interval (CI): 1.195–2.137], poor performance status (HR, 3.67; 95% CI: 2.337–5.770), advanced stage (HR, 2.85; 95% CI: 1.927–4.223), low FEV1 (%) (HR, 0.99; 95% CI: 0.985–0.997), and dyspnea of mMRC grade 2 or higher (HR, 1.84; 95% CI: 1.452–2.339) were associated with shorter survival (Table 3).
We investigated the prognostic role of dyspnea according to the presence and degree of dyspnea in patients with NSCLC. The present study found the presence of dyspnea and pulmonary function parameters of patients with lung cancer to be significantly associated with survival outcomes.
To investigate the prognostic role of dyspnea in patients with lung cancer, most researchers have concentrated on the short-term morbidity of dyspnea after surgery, radiation, and chemotherapy151617 or among long term survivors, not newly diagnosed patients.918 In the current study, we focused on dyspnea at initial presentation in patients with lung cancer. In addition, we measured degree of dyspnea more accurately using the mMRC scale. Simultaneously, we investigated objective parameters of lung function through pulmonary function tests. In doing so, our study found dyspnea to be associated with clinical outcomes and to hold prognostic value.
Recently, Denehy, et al.19 proposed the prognostic role of body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index in inoperable NSCLC. In the study, which included mMRC and FEV1 (%) as parameters of dyspnea and airflow obstruction, respectively, the BODE index was found to be a strong independent predictor of survival in inoperable NSCLC beyond traditional risk factors. Indeed, the BODE index is a multidimensional and effective scoring system to predict clinical outcomes in COPD.20 Among the variables that compose the BODE index, mMRC is an important factor to assess degree of dyspnea and QOL of patients with COPD, even in the GOLD classification, which predicts mortality and patient clinical outcomes.13 In the present study, we showed dyspnea and low FEV1 (%) to be significantly associated with patient prognosis in univariate and multivariate analysis. Our study showed consistent results with Denehy's previous findings in which dyspnea and reduced pulmonary function suggested a poor prognosis in patients with lung cancer. Therefore, individualized and more delicate treatments are required in these patients.
Additionally, we investigated the prevalence of COPD among patients who had dyspnea. Herein, 56% of patients were classified as COPD by the GOLD criteria, and severe or very severe COPD (GOLD 3 and 4) patients comprised up to 20%. These results showed that the prevalence and severity of COPD in patients with NSCLC is higher than that in the general population. Similar results were observed in research by Hashimoto, et al.21 in Japan. However, in clinical settings, dyspnea has not come into the spotlight, and is even ignored sometimes. Zhang, et al.22 reported COPD is substantially underdiagnosed and undertreated in a hospitalized lung cancer population. Especially, non-respiratory doctors had a lower diagnostic rate and a lack of better treatment for exacerbation of COPD than respiratory physicians. Dyspnea in patients with lung cancer is an important clinical sign that could be accompanied by COPD. To improve patient outcomes and QOL, surveillance of the existence of COPD and proper management, such as smoking cessation and using inhalers, should be undertaken when clinicians diagnose lung cancer in patients with dyspnea at initial presentation. Nevertheless, additional prospective study will be needed to delineate the effects of appropriate monitoring and management on patients with lung cancer and COPD.
There were a few limitations in this study. First, we were unable to obtain questionnaires from all of the patients in the study population. Before 2008, the mMRC scale was not used frequently to evaluate degree of dyspnea in patients with lung cancer in our hospital. To reduce predictable statistical errors due to the missing data, we used a linear regression model for imputing the missing values of mMRC. Also, since we consecutively gathered mMRC scores from most patients after 2008, we considered that these problems caused few statistical errors. Second, we did not describe other pulmonary function parameters, such as inspiratory capacity and functional residual capacity, which are closely associated with the severity of dyspnea.23 We failed to obtain these parameters at the initial course of data acquisition. Third, we did not record the exact cause of death in most patients. Thus, we assessed all-cause mortality instead of NSCLC-specific mortality. Finally, our study was conducted in a single institution retrospectively. However, we consecutively gathered patient data from a well-constructed, prospective lung cancer database, and we used validated questionnaires and spirometry to assess degree of dyspnea more precisely and objectively.
In conclusion, this study showed that the prevalence of COPD and reduced pulmonary function are higher in patients with lung cancer who complain of dyspnea at initial presentation than in those without dyspnea. The existence of dyspnea and low FEV1 (%) were significantly associated with poor prognosis. Additionally, the mMRC scale was found to be an independent factor affecting patient survival. Therefore, clinicians should pay more attention to dyspnea and investigate the existence of COPD to improve patient outcomes and QOL.
Figures and Tables
Fig. 1
Distribution of patients with dyspnea according to modified Medical Research Council (mMRC) scale.
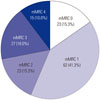
Fig. 2
Prevalence of chronic obstructive pulmonary disease in patients with dyspnea. FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease.

Fig. 3
Kaplan-Meier survival curve of patients according to dyspnea (A) and modified Medical Research Council (mMRC) scale (B).

Table 1
Patient Characteristics

Table 2
Comparisons of Pulmonary Function between Patients with Dyspnea and Those without Dyspnea

Table 3
Prognostic Factors for NSCLC by Multivariate Logistic Regression Analysis

References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

2. Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013; 14:1165–1174.

3. National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.

4. Walters S, Maringe C, Coleman MP, Peake MD, Butler J, Young N, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax. 2013; 68:551–564.

5. Carlson LE, Waller A, Groff SL, Bultz BD. Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems--secondary outcomes of a randomized controlled trial. Psychooncology. 2013; 22:1880–1888.

6. Blanchon F, Grivaux M, Asselain B, Lebas FX, Orlando JP, Piquet J, et al. 4-year mortality in patients with non-small-cell lung cancer: development and validation of a prognostic index. Lancet Oncol. 2006; 7:829–836.

7. Vermaelen K, Brusselle G. Exposing a deadly alliance: novel insights into the biological links between COPD and lung cancer. Pulm Pharmacol Ther. 2013; 26:544–554.

8. Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009; 34:380–386.

9. Sarna L, Evangelista L, Tashkin D, Padilla G, Holmes C, Brecht ML, et al. Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest. 2004; 125:439–445.

10. Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995; 12:199–220.

11. Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JC, Chu DT, et al. Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). J Thorac Oncol. 2011; 6:1872–1880.

12. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002; 121:1434–1440.

13. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187:347–365.

14. Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001; 27:85–96.
15. Charpidou AG, Gkiozos I, Tsimpoukis S, Apostolaki D, Dilana KD, Karapanagiotou EM, et al. Therapy-induced toxicity of the lungs: an overview. Anticancer Res. 2009; 29:631–639.
16. De Ruysscher D, Dehing C, Yu S, Wanders R, Ollers M, Dingemans AM, et al. Dyspnea evolution after high-dose radiotherapy in patients with non-small cell lung cancer. Radiother Oncol. 2009; 91:353–359.

17. Foroulis CN, Lioulias AG, Papakonstantinou C. Simple laboratory parameters which can determine the clinical state of patients after pneumonectomy for lung cancer. J Thorac Oncol. 2009; 4:55–61.

18. Feinstein MB, Krebs P, Coups EJ, Park BJ, Steingart RM, Burkhalter J, et al. Current dyspnea among long-term survivors of early-stage non-small cell lung cancer. J Thorac Oncol. 2010; 5:1221–1226.

19. Denehy L, Hornsby WE, Herndon JE 2nd, Thomas S, Ready NE, Granger CL, et al. Prognostic validation of the body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index in inoperable non-small-cell lung cancer. J Thorac Oncol. 2013; 8:1545–1550.

20. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004; 350:1005–1012.

21. Hashimoto N, Matsuzaki A, Okada Y, Imai N, Iwano S, Wakai K, et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm Med. 2014; 14:14.

22. Zhang J, Zhou JB, Lin XF, Wang Q, Bai CX, Hong QY. Prevalence of undiagnosed and undertreated chronic obstructive pulmonary disease in lung cancer population. Respirology. 2013; 18:297–302.

23. O'Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012; 141:753–762.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download