Abstract
Among intracranial meningiomas, falcotentorial meningiomas, occurring at the junction of the falx cerebri and tentorial dural folds, are extremely rare. Because of their deep location, they are surrounded by critical structures, and have been regarded as one of the most challenging lesions for surgical treatment. In this study, we describe our surgical strategy for falcotentorial meningiomas and provide a review of our experience.
Meningiomas arising from the falcotentorial junction are rare, and constitute about 1% of all intracranial meningiomas.123 Two types of meningiomas can occupy the pineal region: the first type is the velum interpositum meningioma, while the second type is the falcotentorial meningioma. There has been some controversy concerning the definition of falcotentorial meningiomas. However, it is generally accepted that falcotentorial meningiomas arise from the dura of the tentorium cerebelli and posterior part of the falx. These meningiomas have dural attachment, and secondarily invade the pineal region. Meanwhile, velum interpositum meningiomas arise from the velum interpositum, the double layer of pia mater that forms the roof of the third ventricle, and are free lying in the third ventricle without dural attachment.4567 Falcotentorial meningiomas are mainly supplied by the tentorial branch of the meningohypophyseal trunk. However, most velum interpositum meningiomas are distinguished from falcotentorial meningiomas in that they receive their main blood supply from the medial posterior choroidal arteries.189
Of the 2983 meningiomas we encountered from October 2001 to January 2014, we operated on 11 patients with falcotentorial meningiomas, which accounted for about 0.3% of all intracranial meningiomas (Table 1). Preoperatively, all patients underwent magnetic resonance imaging (MRI) and cerebral angiography to determine the appropriate approach trajectory and craniotomy size.
Our main surgical approach was an occipital transtentorial with or without transfalcine approach. Moreover, we favored the three quarter prone position, except in one case, since we believed this position to be safer, providing lower risk of air embolism during the prolonged operative time. In patient positioning, airway patency and jugular venous pressure are the most important factors determining positive outcomes. Therefore, we were very careful to maintain an adequate airway and venous pressure when we elevated, rotated, and flexed the patient's neck.
For determination of bone flap size and location, as well as for identifying which side of the patient was to be upward, we thoroughly reviewed preoperative imaging studies. The location, size, and growth direction of the tumor were all important factors in determining the surgical approach. We compared those factors with surrounding normal brain parenchyma using a three-dimensional (3D) concept with the help of 3D simulation software (3D volume viewer version 1.2.3, released 14th August 2007, RMR systems, East Anglia, UK, www.rmrsystems.co.uk). The approach site was determined based on the shortest pathway from normal brain cortex to the tumor, and the patient's position was set so that the approach axis was parallel to the microscopic view. An occipital bone flap was made over the main bulk of the tumor considering possible exploration area through axis for surgical access. We could remove the tumor totally via a single, relatively small bone flap (Fig. 1). Craniotomy sizes are summarized in Table 2.
At the early stage of surgery, cerebrospinal fluid (CSF) drainage was imperative in most cases. With the aid of a navigation system, CSF was drained through the catheter into the ventricle or cisternal space. In half of the cases, the gravity-dependent position was helpful for gentle retraction of the occipital lobe.
To understand the relationship between the tumors and deep venous structures, pre-operative imaging studies, including thin-sectioned T2-weighted MRI and cerebral angiography with late venous phases, were performed. The vein that drained from the tumor was coagulated with minimal cerebral blood hemodynamic compromise. However, throughout the whole surgery, we tried to preserve physiologic veins. After central debulking of the tumor mass, we predicted the exact course of the deep venous structures and proceeded to remove the tumor, while maintaining the arachnoidal dissection plane. Gradual removal of the tumor in a piecemeal fashion allowed us to identify and save the deep venous structures around the tumor.
In cases in which the tentorium and falx had acted as an obstacle to the tumor, we made incisions on the tentorium and falx. In one case, we ligated and cut the transverse sinus on the non-dominant side to acquire wide exposure of the tumor (Fig. 2). In that case, although we divided the non-dominant transverse sinus, the patient had experienced complications of delayed occipito-cerebellar hemorrhage. The possible adverse effects of the transverse sinus ligation and resection will be discussed later.
Five of these patients were men and six were women, with a mean age of 52.9 years (ranging from 32 to 72 years). The mean follow-up period was 35 months (ranging from 3 to 150 months). The main symptoms included headache in four patients, visual field defects in three patients, and gait disturbance in four patients. The average duration of symptoms was 10.3 months. The mean Karnofsky Performance Scale scores in the preoperative phase and at last follow-up were 84.5 (range, 70 to 90) and 91.8 (60 to 100), respectively. The clinical features of the 11 patients are presented in Table 1.
Like other intracranial meningiomas, the margins of the tumors were relatively distinct from surrounding brain tissues on gadolinium-enhanced T1-weighted images. The average dimensions of the tumors were 4.1×3.4×4.6 cm (anteroposterior×trans-verse×vertical). Cerebral edema around the tumor was found in four patients, and preoperative hydrocephalus was noted in two patients.
Using the classification that Asari, et al.10 published in 1995, the growth directions of the tumors were categorized into four types. There were four anterior-type tumors, two superior-type tumors, four inferior-type tumors, and one posterior-type tumor. Using classification published by Bassiouni, et al.,1 there were four type I, four type II, two type III, and one type IV tumors (Table 2). Preoperative and postoperative coronal T1-weighted contrast-enhanced magnetic resonance images of all 11 patients are shown in Fig. 3.
A summary of the preoperative angiographic findings is presented in Table 3. Considering the benefit-to-risk ratio of preoperative embolization, the embolization procedure was only performed on three patients.
The operative results are summarized in Table 1. Ten patients had gross total resection (Simpson grade 1) confirmed by a postoperative MRI study, while one patient had subtotal resection (Simpson grade 4). There was one case of recurrence where subtotal resection was performed. That patient underwent gamma knife surgery at 6 months after the operation, and there was no evidence of regrowth after 12 months of follow-up.
There were no cases of surgery-related mortality in our series. Most preoperative symptoms (9 of 11 cases in this series) were improved postoperatively. However, one patient, in whom we performed transverse sinus ligation and cutting, experienced delayed occipito-cerebellar hemorrhage that required additional ventricular drainage and an extended recovery time. Moreover, this was the only patient to receive placement of a post-surgical ventriculoperitoneal shunt. One other patient experienced a posterior cerebral artery infarction resulting in visual field defects. All other complications, including the visual field defect and diplopia, were transient and either resolved or improved over several postoperative weeks.
The average age of our patients at the time of operation was 52.9 years and this was similar to the reported average ages of patients with meningiomas at all locations.1112 Furthermore, we could not find gender predominance in our series (five male patients, six female patients).
In general, cortical blindness is the main postoperative problem encountered via the occipito-transtentorial approach, and this is likely due to prolonged retraction of the occipital lobe. However, many studies have reported improvements in patient vision on discharge and, further, that vision often recovers to preoperative baseline levels on follow-up. In our series, we frequently relaxed the occipital lobe during operations, and no patient complained of visual field deterioration during their normal daily activities.
Commonly reported symptoms of falcotentorial meningiomas include headache, visual deficits, gait disturbances, mental deterioration, and cognitive impairment.210 Our findings were similar to these previous reports. Ataxic gait is a common result of an inferior extension of the tumor into the cerebellum.5 In our series, four patients had gait disturbances, and all of their tumor locations and growth directions were inferior or posterior. Upward gaze palsy, a component of Parinaud's syndrome, occurs less frequently in falcotentorial meningiomas than other pineal region tumors.1314 Asari, et al.10 explained that this is the falcotentorial meningioma mainly exists in the cistern and grows slowly so that there is less compression and destruction of the periaqueduct and posterior part of the third ventricle. In our series, there was no symptom of upward gaze palsy.
Various feeding arteries have been reported in falcotentorial meningiomas. Feeding branches from the internal carotid artery include branches of the meningohypophyseal trunk (especially the Bernasconi-Cassinari artery), inferolateral trunk, and anterior choroidal artery. Moreover, there have been some reports that the feeding artery involves ophthalmic artery branches and/or the muscular branch of the vertebral artery.101516
Several surgical approaches have been described to treat falcotentorial meningiomas. These include infratentorial supracerebellar, suboccipital, occipital transtentorial, and combined supratentorial and infratentorial approaches.239 Nowadays, occipital transtentorial and infratentorial supracerebellar approaches are regarded as the standard techniques to treat pineal-region meningiomas.
There are two main issues in treating falcotentorial meningiomas. One is selecting the surgical approach, which includes design of the bone flap. The other main issue is whether main venous structures will be sacrificed for a radical tumor resection. In all of our cases, we tried to make an adequately sized bone flap, even when the tumor was quite large (Fig. 1D). Some authors have insisted on performing wide craniotomies for large falcotentorial meningiomas. For example, Quiñones-Hinojosa, et al.8 described a bilateral occipital transtentorial/transfalcine approach for large falcotentorial meningiomas. They ligated and cut the transverse sinus after checking the patency of the occluded sinus, and used permanent aneurysmal clips to ligate the vein of Galen when the straight sinus was occluded. The area above and below the tentorium can provide wide exposure and reduce occipital lobe retraction during prolonged operation times. Moreover, this approach may allow surgeons some form of intraoperative flexibility in terms of their surgical plan.
It should be noted that we do not suggest routine application of wide craniotomies, such as the combined supratentorial and infratentorial approach. This is because wide craniotomies may increase the total amount of bleeding, prolong the operation time, and increase the risk of cerebral cortex injury. Moreover, it is possible to completely remove huge falcotentorial meningiomas without neurological deficit via relatively small craniotomies. In the current set of craniotomies, a catheter for CSF drainage was inserted into the ventricle or cisternal space through the safest area in each patient. We also designed small craniotomies through which the possible access area covered the entire tumor territory. Thus, if a CSF drain is possible, then appropriately designed small craniotomies are sufficient to achieve complete tumor resection without cortex injury.
There are some reports that have described usage of ligation and sectioning of the transverse sinus with or without reanastomosis. 917 Although many authors have reported safe ligation of the transverse-sigmoid sinus, some complications have been described.1819 In one of our cases, in which we ligated the non-dominant transverse sinus, we experienced delayed occipito-cerebellar hemorrhaging and a prolonged patient recovery period (Fig. 2). Therefore, from our experience, every venous structure should be preserved even if they seem to lack significant function. This will help prevent complications associated with delayed lobar parenchymal hemorrhage that can be attributed to venous infarction.
In conclusion, surgical approaches should be tailored to each patient according to the origin and direction of tumor growth, feeding arteries, and the surrounding venous drainage system. In our series, we found that a relatively small craniotomy was sufficient to completely remove each tumor. Moreover, we found that the most important factors for avoiding surgical complications were to preserve vital deep neurovascular structures, as well as flow through the venous sinuses. Our results showed that falcotentorial meningiomas could be cured via single-stage operations without complications by applying careful perioperative planning and a delicate microsurgical technique.
Figures and Tables
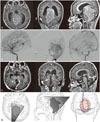 | Fig. 1(A) Preoperative T1-weighted contrast-enhanced magnetic resonance images demonstrating a huge falcotentorial meningioma compressing brain stem and deep venous system. (B) Preoperative angiography showing feeders from meningohypophyseal trunk (the artery of Bernasconi-Cassinari) and posterior meningeal artery from vertebral artery. Venous phase shows occlusion of inferior sagittal sinus and basal vein of Rosenthal. (C) Postoperative T1-weighted contrast-enhanced magnetic resonance images revealing complete tumor removal. (D) Artist's drawing showing possible access area through the relatively small bone flap. View in axial plane, sagittal plane and posterior view. |
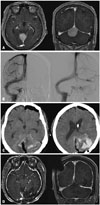 | Fig. 2(A) Preoperative T1-weighted contrast-enhanced magnetic resonance images demonstrating a falcotentorial meningioma abutting both transverse sinus. (B) Preoperative angiography showing right side dominant transverse sinus. (C) Postoperative CT scan showing delayed occipito-cerebellar hemorrhage. (D) Follow up T1-weighted contrast-enhanced magnetic resonance images showing complete tumor removal. |
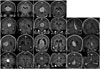 | Fig. 3Total 11 cases' preoperative and postoperative coronal T1-weighted contrast-enhanced magnetic resonance images showing gross total tumor removal in 10 out of 11 cases. |
Table 1
Summary of 11 Patients with Falcotentorial Meningioma

Table 2
Summary of Tumor Locations and Surgical Approach
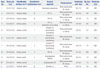
| Case No. | Tumor size (cm) | Classification by Asari, et al.10 | Classification by Bassiouni, et al.1 | Surgical approach | Patient position | Craniotomy size* (cm) | Op. time (hr) | Blood loss (mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.3×3.1×3.2 | Anterior, central | I | Bioccipital transtentorial | Three quarter prone, Rt. side up | 6.5×7.2 | 6.0 | 300 |
| 2 | 3.4×2.7×3.1 | Superior, lateral | III | OTT, Lt. | Three quarter prone, Rt. side up | 4.8×3.6 | 6.5 | 350 |
| 3 | 5.0×4.6×4.6 | Superior, lateral | III | OTT, Rt. | Three quarter prone, Lt. side up | 3.2×3.6 | 7.2 | 350 |
| 4 | 3.3×2.8×3.7 | Inferior, central | II | OTT, Lt. (transverse sinus cutting) | Three quarter prone, Lt. side up | 3.9×5.5 | 6.4 | 400 |
| 5 | 2.5×2.2×2.5 | Anterior, central | I | Occipital, Rt. (transtentorial, transfalcine) | Three quarter prone, Lt. side up | 4.6×5.1 | 5.7 | 630 |
| 6 | 4.2×4.1×4.1 | Anterior, central | I | Occipital, Lt. (transtentorial, transfalcine) | Three quarter prone, Rt. side up | 6.5×4.3 | 10.0 | 450 |
| 7 | 4.5×4.6×4.8 | Inferior, central | II | Occipital, Rt. (transtentorial, transfalcine) | Three quarter prone, Rt. side up | 5.9×7.8 | 7.5 | 1200 |
| 8 | 6.3×5.0×9.3 | Inferior, central | II | OTT, Rt. | Three quarter prone, Rt. side up | 4.7×4.1 | 13.0 | 1500 |
| 9 | 3.8×2.9×3.9 | Inferior, central | II | Occipitoparietal, bilateral | Three quarter prone, Lt. side up | 6.5×5.2 | 7.5 | 300 |
| 10 | 2.9×2.8×3.1 | Anterior, central | I | Occipitoparietal, Rt. | Three quarter prone, Rt. side up | 3.4×4.4 | 7.5 | 650 |
| 11 | 5.2×2.9×8.1 | Posterior, lateral | IV | Occipital, Lt. | Prone | 6.8×7.8 | 9.5 | 800 |
Table 3
Summary of Preoperative Angiographic Findings

ACA, anterior cerebral artery; BVR, basal vein of Rosenthal; GVG, great vein of Galen; ICA, internal cerebral artery; ISS, inferior sagittal sinus; MHT, meningohypophyseal trunk; MMA, middle meningeal artery; PCA, posterior cerebral artery; PMA, posterior meningeal artery; SSS, superior sagittal sinus.
*The first line is the main feeder, †The marginal tentorial branch of MHT is the Bernasconi-Cassinari artery.
ACKNOWLEDGEMENTS
The authors would like to thank Dong-Su Jang, MFA (Medical Illustrator, Seoul, Korea) for his help with the illustrations.
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2014 (6-2014-0060).
References
1. Bassiouni H, Asgari S, König HJ, Stolke D. Meningiomas of the falcotentorial junction: selection of the surgical approach according to the tumor type. Surg Neurol. 2008; 69:339–349.

2. Okami N, Kawamata T, Hori T, Takakura K. Surgical treatment of falcotentorial meningioma. J Clin Neurosci. 2001; 8:Suppl 1. 15–18.

3. Raco A, Agrillo A, Ruggeri A, Gagliardi FM, Cantore G. Surgical options in the management of falcotentorial meningiomas: report of 13 cases. Surg Neurol. 2004; 61:157–164.

4. Obrador S, Soto M, Gutierrez-Diaz JA. Surgical management of tumours of the pineal region. Acta Neurochir (Wien). 1976; 34:159–171.

5. Rozario R, Adelman L, Prager RJ, Stein BM. Meningiomas of the pineal region and third ventricle. Neurosurgery. 1979; 5:489–485.

6. Sachs E Jr, Avman N, Fisher RG. Meningiomas of pineal region and posterior part of 3d ventricle. J Neurosurg. 1962; 19:325–331.
7. Nowak A, Dziedzic T, Czernicki T, Kunert P, Marchel A. Falcotentorial and velum interpositum meningiomas: two distinct entities of the pineal region. Neurol Neurochir Pol. 2014; 48:397–402.

8. Quiñones-Hinojosa A, Chang EF, Chaichana KL, McDermott MW. Surgical considerations in the management of falcotentorial meningiomas: advantages of the bilateral occipital transtentorial/transfalcine craniotomy for large tumors. Neurosurgery. 2009; 64:5 Suppl 2. 260–268.

9. Sekhar LN, Goel A. Combined supratentorial and infratentorial approach to large pineal-region meningioma. Surg Neurol. 1992; 37:197–201.

10. Asari S, Maeshiro T, Tomita S, Kawauchi M, Yabuno N, Kinugasa K, et al. Meningiomas arising from the falcotentorial junction. Clinical features, neuroimaging studies, and surgical treatment. J Neurosurg. 1995; 82:726–738.
11. Kuratsu J, Ushio Y. Epidemiological study of primary intracranial tumors: a regional survey in Kumamoto Prefecture in the southern part of Japan. J Neurosurg. 1996; 84:946–950.

12. Rohringer M, Sutherland GR, Louw DF, Sima AA. Incidence and clinicopathological features of meningioma. J Neurosurg. 1989; 71(5 Pt 1):665–672.

13. Piatt JH Jr, Campbell GA. Pineal region meningioma: report of two cases and literature review. Neurosurgery. 1983; 12:369–376.

14. Papo I, Salvolini U. Meningiomas of the free margin of the tentorium developing in the pineal region. Neuroradiology. 1974; 7:237–243.

15. Ameli NO, Armin K, Saleh H. Incisural meningiomas of the falco-tentorial junction. A report of two cases. J Neurosurg. 1966; 24:1027–1030.
16. Nishiura I, Handa H, Yamashita J, Suwa H. Successful removal of a huge falcotentorial meningioma by use of the laser. Surg Neurol. 1981; 16:380–385.

17. Hwang SK, Gwak HS, Paek SH, Kim DG, Jung HW. Guidelines for the ligation of the sigmoid or transverse sinus during large petroclival meningioma surgery. Skull Base. 2004; 14:21–28.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download