Abstract
Purpose
Extremely low birth weight infants (ELBWIs) have a high risk of acquiring cytomegalovirus (CMV) infection via breast milk and consequently developing serious symptoms. We evaluated whether freeze-thawing or pasteurization could prevent postnatal CMV infection transmitted through breast milk in ELBWIs.
Materials and Methods
Medical records of 385 ELBWIs with whole milk feeding, and freeze-thawed or pasteurized breast milk feeding were reviewed retrospectively. Postnatally acquired CMV infection was defined as an initial negative and a subsequent positive on follow-up urine CMV DNA polymerase chain reaction screening tests. The incidence, clinical characteristics, symptoms, sequelae, and long-term outcome at corrected age [(CA): 2 years of CMV infection] were analyzed.
Results
While no infant developed CMV infection with whole milk (0/22) or pasteurized breast milk (0/62) feeding, postnatal CMV infection was diagnosed in 8% (27/301) of ELBWIs who were fed freeze-thawed breast milk. Gestational age in the CMV group was significantly lower than the control group. In 82% (22/27) of cases, CMV infection was symptomatic and was associated with increased ventilator days and ≥moderate bronchopulmonary dysplasia (BPD). Neurodevelopmental outcome and growth status at CA 2 years were not different between the study groups. Lower gestational age and freeze-thawed breast milk feeding >60% of total oral intake during the first 8 postnatal weeks were independent risk factors for acquiring postnatal CMV infection. BPD (≥moderate) was the only significant adverse outcome associated with this CMV infection.
The persistent beneficial effects of breast milk ingested in the neonatal intensive care unit (NICU) on growth and developmental outcome and its anti-infectious properties have been reported in extremely low birth weight infants (ELBWIs) whose birth weight is less than 1000 gm.1,2,3 Therefore, breast milk has been considered to be the ideal food, and continued efforts have been made to feed breast milk to all ELBWIs in the NICU.4 However, ELBWIs are at the highest risk of developing severe symptomatic postnatal cytomegalovirus (CMV) infection manifesting as sepsis-like symptoms or pneumonitis.5,6,7 Transmission of CMV through breast milk to ELBWIs has been known to be the primary source of postnatal CMV infection.8 The majority of CMV immunoglobulin (Ig) G seropositive women becomes locally reactivated during lactation, and subsequently excretes CMV in the breast milk without clinical or laboratory signs of systemic infection.2,7,8 Therefore, the risk of postnatal CMV infection might be even greater in ELBWIs born in the country or ethnicity with the high prevalence of CMV IgG seropositive women.9
The efficacy of various methods of CMV inactivation in breast milk, such as freeze-thawing or pasteurization, has not yet been elucidated. Although conventional pasteurization has been known to be effective in limiting CMV transmission through breast milk,10,11,12 such manipulation might severely affect milk quality, thus limiting its benefits.12,13 Freezing breast milk at -20℃ for several days has been found to reduce or destroy viral infectivity in vitro.12,14 Moreover, the nutritional and immunological advantages of breast milk could be preserved with this method.15 In a previous study, however, a certain level of infectivity has been shown to remain, and freeze-thawed breast milk could not completely prevent postnatal CMV infection.14 Therefore, the available data regarding the efficacy of strategies such as freeze-thawing or pasteurization of breast milk in preventing postnatal CMV infection remain largely undetermined, and further studies are needed. In the present retrospective study, we evaluated the efficacy of freeze-thawing and pasteurization in preventing postnatal CMV infection transmitted through breast milk. The symptoms and outcome were described, once infected with CMV, to determine whether postnatal CMV infection acquired through breast milk leads to severe symptoms in ELBWIs.
Data collection was approved by the Institutional Review Board of Samsung Medical Center, and the Institutional Review Board allowed a waiver of informed consent requirements for this retrospective chart review. Medical records of 385 out of 579 ELBWIs admitted to the NICU of Samsung Medical Center in the periods from January 1, 2007 to December 31, 2012 (period I, n=323) and from January 1, 2013 to September 30, 2013 (period II, n=62) were reviewed retrospectively. The details of 194 infants excluded in the analysis are shown in Fig. 1. The study period was arbitrarily divided according to the methods of CMV inactivation in the stored human milk. During period I, each infant was fed with freeze-thawed mother's own breast milk that had been stored at -20℃ for more than 3 days. After an initial data analysis during period I showing that freeze-thawing reduced but did not completely prevent CMV transmission via breast milk,16 our policy was changed to test whether pasteurization could eliminate the acquisition of postnatal CMV infection through breast milk, and, therefore, the infants were fed mother's own breast milk that had been conventionally pasteurized at 63℃ for 30 min during period II.12 We also evaluated whether infants fed pasteurized milk might have an increased risk of several neonatal outcomes by comparing the outcomes with period I because pasteurization may reduce the immunological and clinical benefits of breast milk. Supplemental cow's whole milk for preterm infants (Premie®, Maeil Dairy Co. Ltd., Seoul, Korea) feeding was provided if necessary during the entire study period. Contact isolation guidelines were strictly implemented, and leukocyte depleted and irradiated blood was transfused to prevent acquired CMV infection throughout the whole study period.
Due to the high prevalence of CMV IgG positive mothers in Korea,9 we developed a policy to routinely screen ELBWIs for CMV infection during admission to the NICU in 2007. Congenital CMV infection was ruled out by initial negative CMV screening test with urine CMV DNA polymerase chain reaction (PCR) performed within the first 2 postnatal weeks,17 and the screening test was repeated if the patient was clinically suspicious of CMV infection, or every 2 to 6 weeks until discharge in order to detect postnatal CMV infection. The lowest limit of detection was 360 copies/mL. Postnatally acquired CMV infection was defined as an initial negative and subsequent positive on follow up CMV screening tests. Maternal CMV IgG level was checked only during period II.
The incidence of postnatally acquired CMV infection was assessed throughout the study period. Clinical findings including gestational age (GA), birth weight, Apgar score at 1 and 5 min, gender, small for GA, mode of delivery, premature rupture of membrane (PROM), pregnancy induced hypertension, maternal diabetes mellitus, and antenatal steroid use were analyzed. GA was determined by maternal last menstrual period and modified Ballard test. Small for gestational age was defined when the birth weight was less than the tenth percentile. Respiratory distress syndrome was defined by the requirement of surfactant and ventilator treatment. PROM was positive when the duration of PROM was more than 24 hours. To identify risk factors for the development of postnatally acquired CMV infection, the number and frequency of transfusion during admission and the percentage of breast milk feeding out of total oral intake during the first 8 postnatal weeks were calculated.
Outcome measures including death before discharge, bronchopulmonary dysplasia (BPD) (≥moderate),18 intraventricular hemorrhage (IVH) (≥grade 3),19 periventricular leukomalacia (PVL), necrotizing enterocolitis (≥Bell's stage IIb)20 and retinopathy of prematurity (ROP)21 requiring laser treatment were analyzed in each group. Duration of ventilator care, continuous positive airway pressure, and oxygen therapy were also reviewed respectively.
In the CMV group, clinical and laboratory abnormalities including respiratory deterioration, liver function, and hematologic abnormalities during the 3 weeks after first detection of viruria were assessed. Thrombocytopenia and neutropenia were defined as platelet level <100000/mm3 and absolute neutrophil counts <1000/mm3, respectively. Respiratory deterioration was defined as increased oxygen requirement with an increase in the inspired oxygen fraction ≥0.2 and/or increased mean airway pressure of ≥3 cmH2O. Retinitis and CMV encephalopathy were screened by an ophthalmologist and radiologist, respectively. Symptomatic CMV infection was defined only if other attributable causes that might cause these abnormalities were ruled out.
On follow-up at the corrected age (CA) of 2 years, developmental status such as cerebral palsy, hearing impairment, blindness and Bayley score, growth status, and catch-up growth were assessed.22 Cerebral palsy was defined as a non-progressive central nervous system disorder characterized by abnormal muscle tone in at least one extremity and abnormal control of movement and posture.23 Three groups were defined according to the severity of handicap as follows: group 1, children who could walk independently; group 2, children who could not walk but could sit independently; and group 3, children who could not sit independently (unable to maintain head and trunk control).24 Hearing loss was defined as bilateral impairment requiring hearing aids, and blindness was defined as <20/200 visual acuity. The mental development index and psychomotor development index according to Bayley Scales of Infants Development II,22 assessed at a CA of 24 months, were also recorded. Catch-up growth was defined as achievement of normal (10-90th percentile) height, weight, and head circumference at CA of 2 years.
Data are expressed as mean±standard deviation. Comparisons between categorical variables were performed using the chi square test or Fisher's exact test, and comparisons between continuous variables were evaluated using the Mann-Whitney U test. Logistic regression analysis was performed to compare risk factors and morbidities of postnatal CMV infection. A p-value of <0.05 was considered statistically significant. The SPSS version 17 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
During period I, while none (0/22) developed postnatal CMV infection with whole milk feeding, postnatal CMV infection was diagnosed in 8% (27/301) of ELBWIs fed with freeze-thawed breast milk (Table 1). The incidence of CMV infection rose significantly from 4% (3/70) to 10% (24/231) when fed with ≤60% or >60%, respectively, of freeze-thawed mother's own breast milk out of the total oral intake during the first 8 postnatal weeks. The first detection of CMV PCR DNA in urine occurred at 54±24 postnatal days. CMV was also detected in 11/17 (65%) of the CMV infected ELBWIs' stored breast milk at the first detection of viruria. However, during period II, although the maternal CMV IgG level was positive in 95% (59/62) of cases, none (0/62) developed postnatal CMV infection regardless of whole or breast milk feeding.
Demographic and clinical findings of the study groups during period I and II are shown in Table 2. During period I, GA was significantly lower in the postnatal CMV infection group than the control group. During period II, birth weight and Apgar score at 1 and 5 min were significantly lower compared to the control group in period I. Other variables including number and frequency of blood transfusion were not significantly different between the study groups.
In the present study, 82% (22/27) of the CMV infection group were symptomatic within 3 weeks after the first detection of viruria. Thrombocytopenia (63%, 17/27) was the most common abnormality, followed by neutropenia (44%, 12/27), direct hyperbilirubinemia of >2 mg/dL (30%, 8/27), increased liver enzymes (26%, 7/27), and increased respiratory support (19%, 5/27). Neither retinitis nor encephalopathy was detected.
Adverse outcomes of the study groups during period I and II are shown in Table 3. During period I, the incidence of BPD (≥moderate) and duration of ventilator care were significantly higher in the CMV group than the control group. During period II, duration of oxygen was significantly lower than period I. Other adverse outcomes such as ROP requiring surgery, NEC (≥stage II), IVH (≥grade III), and cystic PVL were not significantly different between the study groups.
Neurodevelopmental outcome and growth status at the CA of 2 years in the postnatal CMV infection and control group infants during period I are shown in Table 4. Neurodevelopmental outcome and growth status were not significantly different between the study groups, and neither hearing loss nor blindness was reported in the CMV group.
In multiple logistic regression analysis, lower GA per week and breast milk feeding >60% during the first 8 postnatal weeks were two independent risk factors for acquiring postnatal CMV infection (Table 5).
For adverse outcomes, development of BPD (≥moderate) was the only adverse outcome significantly associated with postnatal CMV infection (Table 6).
The finding that CMV infection occurred only with breast milk but not with whole milk feeding supports the findings of previous studies showing that breast milk is the major route of postnatal CMV transmission, especially in ELBWIs.5,25,26,27,28 However, as breast milk has been considered the ideal food for preterm newborns because of its nutritional benefits and anti-infectious components,1,2,3 withholding breast milk and routine use of whole milk feeding, especially in the ELBWIs, is not acceptable in clinical practice. The majority of CMV IgG seropositive mothers reactivate CMV during lactation, and shed the virus into the breast milk.12,29 In the present study, CMV DNA was detected in 70% stored breast milk at the first detection of viruria. As the transmission of CMV through breast milk has been observed only when an infant is fed CMV IgG positive breast milk with viral shedding,25 the postnatal acquisition of CMV infection via breast milk could be prevented by simply avoiding the feeding of CMV seropositive breast milk. However, the CMV seropositive prevalence during period I was not routinely screened and thus unavailable. Therefore, considering the very high 95% CMV seropositive prevalence observed during period II in the present study, it would be virtually impossible to screen for and provide noninfectious CMV IgG negative breast milk to prevent CMV infection through breast milk in the clinical setting. Consequently, the development of other effective measures that could prevent CMV transmission through breast milk, especially in ELBWIs is an urgent issue.
In the present study, a positive correlation was observed between the acquisition of postnatal CMV infection and the amount of freeze-thawed breast milk ingested. A significantly higher (10%) incidence of postnatal CMV infection was observed when fed >60% freeze-thawed breast milk out of the total oral intake compared with a 4% when fed ≤60% breast milk out of the total oral intake during the first 8 postnatal weeks. Furthermore, >60% freeze-thawed breast milk feeding out of the total oral intake during the first 8 weeks was the independent risk factor for postnatal acquisition of CMV infection. These findings suggest that increased cumulative viral load in freeze-thawed breast milk plays a critical role in the acquisition of postnatal CMV infection via breast milk.30
Lower GA in preterm infants has been known to be an independent risk factor for the acquisition of postnatal CMV infection5,8 probably due to a reduced trans-placental transfer of antibodies before GA of 28 weeks.31 In the present study, the average GA of 24 weeks in the CMV infected ELBWIs was significantly lower compared to the 26 weeks of the control group. Furthermore, in addition to breast feeding >60% of the total oral intake, the risk of postnatal CMV infection increased significantly with each week of decreasing GA. We assume that the absence of prenatally acquired anti-CMV antibodies during early GA in combination with the presence of CMV in breast milk is the most plausible explanation for the increased risk of postnatal acquisition of CMV infection via breast milk among ELBWIs with lower GA.32
Previous reports have shown that extremely immature infants are at the greatest risk for developing serious symptoms and sequelae following postnatal acquisition of CMV through the ingestion of breast milk.2,6,8,25,33,34,35 In the present study, the rate of symptomatic CMV infection was higher than previous studies2,8,33,36 because the enrolled infants were with much lower GA and birth weight compared with previous study, and the majority of CMV infected ELBWIs developed serious symptoms of CMV infection such as thrombocytopenia, neutropenia, hepatopathy, and respiratory deterioration. Moreover, symptomatic CMV infection was associated with significantly increased incidence of ≥moderate BPD. In concordance with our data showing that the majority of CMV infected more immature ELBWIs developed serious symptoms, Mehler, et al.37 reported that all cases of CMV infected infants at 22-24 weeks' gestation presented with thrombocytopenia, and 55% developed sepsis-like symptoms and signs of respiratory failure. Overall, these findings suggest that our data of high percentage of symptomatic CMV infection might be attributable to the lower GA, i.e., more immaturity even within ELBWIs. Yeager, et al.38 reported that fatal or serious symptoms of transfusion acquired CMV infection were observed only in preterm infants born to seronegative mothers. Therefore, one possible explanation for this positive correlation of lower GA with the development of symptomatic CMV infection and sequelae in ELBWIs born even to seropositive mothers might be attributable to the lack of prenatal trans-placental transfer of anti-CMV IgG during early GA in ELBWIs.32 Given the protective effects of CMV IgG against symptomatic CMV infection, further studies will be necessary to determine whether the administration of CMV-specific hyperimmune globulin to ELBWIs could prevent postnatal CMV infection acquired via breast milk and/or treat already established CMV disease, especially in ELBWIs of ≤24 weeks' gestation.39
A few data exist on the long-term outcome of postnatal CMV infection acquired through breast milk in ELBWIs. In the present study, the majority of CMV infected ELBWIs were symptomatic and associated with adverse outcomes such as ≥moderate BPD. Nevertheless, the neurodevelopmental outcome and growth status of the CMV infection group were not significantly different compared to the control group at a CA of 2 years. Moreover, no hearing loss was observed in the postnatal CMV infection group. Overall, these results suggest that CMV infection acquired postnatally via breast milk in ELBWIs does not seem to be associated with long-term sequelae such as impaired development or hearing loss.40
In in vitro studies, freezing breast milk at -20℃ overnight, for more than 72 hours and 7 days reduced the CMV titer by 90%, 99%, and 100%, respectively.10,11 Moreover, freeze-thawing preserved the nutritional and immunologic components of the breast milk.15 However, although the transmission rate in the in vivo studies using freeze-thawed breast milk was significantly reduced to 6-22%33,41,42,43 compared to 38-65% in studies with native untreated breast milk,25,37,44 freezing could not completely inactivate the infectivity of the breast milk. In the present study, although the majority of infants did not acquire CMV infection, 8% of ELBWIs fed freeze-thawed mother's own breast milk stored at -20℃ for more than 3 days still acquired postnatal CMV infection. Overall, these findings suggest that despite its preservation of the nutritional and immunological benefits of breast milk, freezing breast milk at -20℃ for more than 3 days reduces but does not completely prevent CMV transmission through breast milk. Our finding of no postnatal acquisition of CMV when fed conventionally pasteurized breast milk at 63℃ for 30 min indicates that pasteurization could completely inactivate CMV and eradicate CMV transmission through breast milk even in ELBWIs of seropositive mothers.45 Moreover, no significant differences in the outcomes between the period I and II indicates that pasteurization did not significantly reduce the immunological and clinical benefits of breast milk. However, concerns exist regarding the nutritional and immunological quality of breast milk after Holder pasteurization.45 Therefore, further studies will be necessary to determine whether other measures such as short term pasteurization at 72℃ for 10 sec could effectively inactivate CMV without destroying the nutritional and immunological components of breast milk.46
Although pasteurization was shown to eradicate the postnatal acquisition of CMV infection via breast milk in this study, the duration of pasteurization necessary for ELBWIs born to seropositive mothers to prevent the transmission of CMV through breast milk is not yet known. The viral reactivation and shedding into breast milk might begin in the first week, reach a peak at 4-8 weeks, and rapidly decline from 9-12 postnatal weeks.12 These findings suggest that feeding of colostrum might not be quite infectious regardless of freeze-thawing or pasteurization, and our finding of first detection of viruria at an average of 54 postnatal days coincides with the period of maximum viral shedding into breast milk. Taken together, these findings suggest that pasteurization starting from second week after birth for at least the first 8 postnatal weeks to the extremely preterm infants at the limit of viability will be necessary to effectively prevent CMV transmission through breast milk.
In summary, postnatally acquired CMV infection via breast milk was associated with serious symptoms and increased incidence of BPD, especially in ELBWIs with lower GA, and conventional pasteurization but not freeze-thawing of breast milk completely prevented the transmission of CMV through breast milk.
Figures and Tables
 | Fig. 1Diagram showing enrollment and follow up of study patients during period I and II. ELBWI, extremely low birth weight infant; CMV, cytomegalovirus. |
Table 1
Incidence of Postnatal Cytomegalovirus Infection during Period I and II

Table 2
Clinical Characteristics of the Control and Cytomegalovirus Infection Group during Period I and II
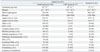
Table 3
Adverse Outcomes of the Control and CMV Infection Group during Period I and II
CMV, cytomegalovirus; BPD, bronchopulmonary dysplasia; CPAP, continuous positive airway pressure; ROP, retinopathy of prematurity; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; CA, corrected age.
Data are expressed as mean±SD.
*p value<0.05 compared to control in period I.
Table 4
Outcome at Corrected Age of 24 Months in the Control and Cytomegalovirus Infection Group
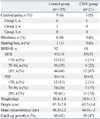
Table 5
Adjusted Odds Ratios of Risk Factors for Developing CMV Infection
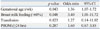
| p value | Odds ratio | 95% CI | |
|---|---|---|---|
| Gestational age (/wk) | 0.043 | 1.36 | 1.07-1.72 |
| Breast milk feeding (>60%) | 0.048 | 3.40 | 1.01-11.72 |
| Transfusion | 0.823 | 1.27 | 0.14-11.02 |
| PROM (≥24 hrs) | 0.287 | 1.60 | 0.67-3.85 |
Notes
References
1. Lawrence RA. The evidence for growth standards and iron in moderation and exclusive breastfeeding. Breastfeed Med. 2006; 1:205–206.

2. Hamprecht K, Maschmann J, Jahn G, Poets CF, Goelz R. Cytomegalovirus transmission to preterm infants during lactation. J Clin Virol. 2008; 41:198–205.

3. Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Higgins RD, Langer JC, et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007; 120:e953–e959.

4. Dougherty D, Luther M. Birth to breast--a feeding care map for the NICU: helping the extremely low birth weight infant navigate the course. Neonatal Netw. 2008; 27:371–377.

5. Nijman J, de Vries LS, Koopman-Esseboom C, Uiterwaal CS, van Loon AM, Verboon-Maciolek MA. Postnatally acquired cytomegalovirus infection in preterm infants: a prospective study on risk factors and cranial ultrasound findings. Arch Dis Child Fetal Neonatal Ed. 2012; 97:F259–F263.

6. Numazaki K. Human cytomegalovirus infection of breast milk. FEMS Immunol Med Microbiol. 1997; 18:91–98.

7. Vochem M, Hamprecht K, Jahn G, Speer CP. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J. 1998; 17:53–58.

8. Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001; 357:513–518.

10. Dworsky M, Stagno S, Pass RF, Cassady G, Alford C. Persistence of cytomegalovirus in human milk after storage. J Pediatr. 1982; 101:440–443.

11. Friis H, Andersen HK. Rate of inactivation of cytomegalovirus in raw banked milk during storage at -20 degrees C and pasteurisation. Br Med J (Clin Res Ed). 1982; 285:1604–1605.

12. Hamprecht K, Maschmann J, Müller D, Dietz K, Besenthal I, Goelz R, et al. Cytomegalovirus (CMV) inactivation in breast milk: reassessment of pasteurization and freeze-thawing. Pediatr Res. 2004; 56:529–535.

13. Ewaschuk JB, Unger S, O'Connor DL, Stone D, Harvey S, Clandinin MT, et al. Effect of pasteurization on selected immune components of donated human breast milk. J Perinatol. 2011; 31:593–598.

14. Maschmann J, Hamprecht K, Weissbrich B, Dietz K, Jahn G, Speer CP. Freeze-thawing of breast milk does not prevent cytomegalovirus transmission to a preterm infant. Arch Dis Child Fetal Neonatal Ed. 2006; 91:F288–F290.

15. Schanler RJ. Suitability of human milk for the low-birthweight infant. Clin Perinatol. 1995; 22:207–222.

16. Yoo HS, Shin JH, Jung UJ, Kim JK, Ahn SY, Kim ES, et al. Perinatal cytomegalovirus infection associated with freeze-thawed breast milk feeding in extremely low birth weight infants (<1000 gm). Denver: In SPR (Society for Pediatric Research);2009.
17. Klein JO, Wilson CB, Nizet V, Maldonado YA. Infectious Diseases of the Fetus and Newborn Infant. 7th ed. Philadelphia: Elsevier;2011.
18. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005; 116:1353–1360.

19. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–534.

20. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978; 187:1–7.
21. The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984; 102:1130–1134.
22. Leonard CH, Piecuch RE, Cooper BA. Use of the Bayley Infant Neurodevelopmental Screener with low birth weight infants. J Pediatr Psychol. 2001; 26:33–40.

23. Shankaran S, Johnson Y, Langer JC, Vohr BR, Fanaroff AA, Wright LL, et al. Outcome of extremely-low-birth-weight infants at highest risk: gestational age < or =24 weeks, birth weight < or =750 g, and 1-minute Apgar < or =3. Am J Obstet Gynecol. 2004; 191:1084–1091.

24. Ancel PY, Livinec F, Larroque B, Marret S, Arnaud C, Pierrat V, et al. Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics. 2006; 117:828–835.

25. Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clin Infect Dis. 2001; 33:1998–2003.

26. Nijman J, van Loon AM, de Vries LS, Koopman-Esseboom C, Groenendaal F, Uiterwaal CS, et al. Urine viral load and correlation with disease severity in infants with congenital or postnatal cytomegalovirus infection. J Clin Virol. 2012; 54:121–124.

27. Hayashi S, Kimura H, Oshiro M, Kato Y, Yasuda A, Suzuki C, et al. Transmission of cytomegalovirus via breast milk in extremely premature infants. J Perinatol. 2011; 31:440–445.

28. Okulu E, Akin IM, Atasay B, Ciftçi E, Arsan S, Türmen T. Severe postnatal cytomegalovirus infection with multisystem involvement in an extremely low birth weight infant. J Perinatol. 2012; 32:72–74.

29. Hamprecht K, Witzel S, Maschmann J, Dietz K, Baumeister A, Mikeler E, et al. Rapid detection and quantification of cell free cytomegalovirus by a high-speed centrifugation-based microculture assay: comparison to longitudinally analyzed viral DNA load and pp67 late transcript during lactation. J Clin Virol. 2003; 28:303–316.

30. Jim WT, Shu CH, Chiu NC, Chang JH, Hung HY, Peng CC, et al. High cytomegalovirus load and prolonged virus excretion in breast milk increase risk for viral acquisition by very low birth weight infants. Pediatr Infect Dis J. 2009; 28:891–894.

31. Mussi-Pinhata MM, Pinto PC, Yamamoto AY, Berencsi K, de Souza CB, Andrea M, et al. Placental transfer of naturally acquired, maternal cytomegalovirus antibodies in term and preterm neonates. J Med Virol. 2003; 69:232–239.

32. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012; 2012:985646.

33. Doctor S, Friedman S, Dunn MS, Asztalos EV, Wylie L, Mazzulli T, et al. Cytomegalovirus transmission to extremely low-birthweight infants through breast milk. Acta Paediatr. 2005; 94:53–58.

34. Capretti MG, Lanari M, Lazzarotto T, Gabrielli L, Pignatelli S, Corvaglia L, et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother's milk: a prospective study. J Pediatr. 2009; 154:842–848.

35. Cheong JL, Cowan FM, Modi N. Gastrointestinal manifestations of postnatal cytomegalovirus infection in infants admitted to a neonatal intensive care unit over a five year period. Arch Dis Child Fetal Neonatal Ed. 2004; 89:F367–F369.

36. Arellano-Galindo J, Villanueva-García D, Cruz-Ramirez JL, Yalaupari-Mejìa JP, Uribe-Gutiérrez G, Velazquez-Guadarrama N, et al. Detection and gB genotyping of CMV in Mexican preterm infants in the context of maternal seropositivity. J Infect Dev Ctries. 2014; 8:758–767.

37. Mehler K, Oberthuer A, Lang-Roth R, Kribs A. High rate of symptomatic cytomegalovirus infection in extremely low gestational age preterm infants of 22-24 weeks' gestation after transmission via breast milk. Neonatology. 2014; 105:27–32.

38. Yeager AS, Grumet FC, Hafleigh EB, Arvin AM, Bradley JS, Prober CG. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981; 98:281–287.

39. Snydman DR, Werner BG, Meissner HC, Cheeseman SH, Schwab J, Bednarek F, et al. Use of cytomegalovirus immunoglobulin in multiply transfused premature neonates. Pediatr Infect Dis J. 1995; 14:34–40.

40. Kurath S, Halwachs-Baumann G, Müller W, Resch B. Transmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin Microbiol Infect. 2010; 16:1172–1178.

41. Jim WT, Shu CH, Chiu NC, Kao HA, Hung HY, Chang JH, et al. Transmission of cytomegalovirus from mothers to preterm infants by breast milk. Pediatr Infect Dis J. 2004; 23:848–851.

42. Mussi-Pinhata MM, Yamamoto AY, do Carmo Rego MA, Pinto PC, da Motta MS, Calixto C. Perinatal or early-postnatal cytomegalovirus infection in preterm infants under 34 weeks gestation born to CMV-seropositive mothers within a high-seroprevalence population. J Pediatr. 2004; 145:685–688.

43. Yasuda A, Kimura H, Hayakawa M, Ohshiro M, Kato Y, Matsuura O, et al. Evaluation of cytomegalovirus infections transmitted via breast milk in preterm infants with a real-time polymerase chain reaction assay. Pediatrics. 2003; 111(6 Pt 1):1333–1336.

44. Meier J, Lienicke U, Tschirch E, Krüger DH, Wauer RR, Prösch S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol. 2005; 43:1318–1324.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download