Abstract
Purpose
Copeptin has been considered as a useful marker for diagnosis and prediction of prognosis in heart diseases. However, copeptin has not been investigated sufficiently in hemodialysis patients. This study aimed to investigate the general features of copeptin in hemodialysis and to examine the usefulness of copeptin in hemodialysis patients with left ventricular dysfunction (LV dysfunction).
Materials and Methods
This study included 41 patients on regular hemodialysis. Routine laboratory data and peptides such as the N-terminal of the prohormone brain natriuretic peptide and copeptin were measured on the day of hemodialysis. Body fluid volume was estimated by bioimpedance spectroscopy, and the E/Ea ratio was estimated by echocardiography.
Results
Copeptin increased to 171.4 pg/mL before hemodialysis. The copeptin had a positive correlation with pre-dialysis body fluid volume (r=0.314; p=0.04). The copeptin level decreased along with body fluid volume and plasma osmolality during hemodialysis. The copeptin increased in the patients with LV dysfunction more than in those with normal LV function (218.7 pg/mL vs. 77.6 pg/mL; p=0.01). Receiver operating characteristic curve analysis showed that copeptin had a diagnostic value in the hemodialysis patients with LV dysfunction (area under curve 0.737; p=0.02) and that the cut-off value was 125.48 pg/mL (sensitivity 0.7, specificity 0.8, positive predictive value 0.9, negative predictive value 0.6).
Copeptin is the peptide at the C-terminal of preprovasopressin. Recent studies have demonstrated that copeptin, a surrogate marker for vasopressin, could predict the prognosis of heart failure 1,2,3,4,5 and myocardial infarction.6,7 In addition, it could also be useful in the diagnosis of left ventricular dysfunction (LV dysfunction) and myocardial infarction.6,8
Dialysis patients are usually classified as high-risk for heart diseases. Therefore, the clinical use of copeptin should be considered for dialysis patients. For this, our study aimed to investigate the general features of copeptin in hemodialysis. In addition, we hypothesized that copeptin could be useful in the diagnosis of LV dysfunction in hemodialysis patients. To determine the usefulness of copeptin, our study investigated the difference in copeptin level between patients with normal LV function and those with LV dysfunction.
This study targeted 41 patients who had received hemodialysis regularly for three times per week. When the patients visited the hospital for dialysis, we collected clinical and laboratory data. In particular, serum sodium concentration, plasma osmolality, body fluid volume, and copeptin were measured both before and after dialysis in order to evaluate changes during hemodialysis. Body fluid volume was measured by bioimpedance spectroscopy (Body Composition Monitoring™, Fresenius Medical Care, Bad Homburg, Germany). Copeptin was quantified using an ELISA kit (copeptin: USCNK Life Science Inc., sensitivity 5.7 pg/mL, CV intra-assay<10%, inter-assay<12%).
We also measured the N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) and performed echocardiography to evaluate heart dysfunction. These tests were performed before dialysis. In echocardiography, we measured the E/Ea ratio, which was used to estimate LV end diastolic pressure. We used an NT-proBNP level as a standard to determine LV dysfunction in our study, as the accuracy of an echocardiogram depends on the skill of the performer. We applied an NT-proBNP level of 5300 pg/mL as a threshold for the determination of LV dysfunction according to a study by David, et al.9 in which this value was considered to indicate LV dysfunction in hemodialysis patients.
Copeptin was measured pre-dialysis (pre-copeptin) and post-dialysis (post-copeptin). The value of body fluid excess measured by bioimpedance spectroscopy was presented as the index of overhydration (OH, liter). For example, OH, 1 means that body fluid excess is one liter. The OH value was also measured pre-dialysis (pre-OH) and post-dialysis (post-OH).
The statistical program PASW 18.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The Shapiro-Wilk test was conducted to check for normality. The result showed that the variables had non-normal distributions; therefore, we performed non-parametric tests. Spearman's correlation analysis was used to examine correlations between copeptin and other variables. The Mann-Whitney U test was conducted to investigate the differences between the patients with normal LV function and those with LV dysfunction. Statistical significance was defined as p<0.05.
The average (±standard deviation) age of the patients was 57±10 years. In total, 17 male and 24 female patients were included in the study. Hemodialysis was performed three times per week for 4 hours per session. The average duration of hemodialysis was 2721±2367 days. Diabetes was found in 20 (48.8%) patients, and high blood pressure was found in 36 (87.8%) patients. Three (7.3%) patients had a past history of cardiovascular disease, and four (9.8%) patients had been diagnosed with heart failure. Six (14.6%) patients had a history of stroke. The average Kt/V was 1.44±0.23. Laboratory data [median (interquartile range)] yielded the following values: whole blood hemoglobin 9.7 (9.3, 10.7) g/dL, serum albumin 3.9 (3.8, 4.1) g/dL, calcium 9.0 (8.7, 9.3) mg/dL, phosphorus 6.5 (5.0, 6.8) mg/dL, high-sensitivity C-reactive protein 0.13 (0.06, 0.34) mg/dL, and intact parathyroid hormone 191.2 (111.8, 272.8) pg/mL. NT-proBNP was elevated to 13071 (3984, 35000) pg/mL, and the E/Ea ratio was 5.5 (4.4, 7.2).
Spearman's correlation analysis indicated that pre-copeptin had a significant positive correlation with pre-OH (p=0.04) (Fig. 1). When adjusted for age, sex, plasma osmolality, NT-proBNP, and E/Ea ratio, pre-copeptin still showed a significant positive correlation with pre-OH (p=0.04).
We divided subjects into normal LV function (n=12) and LV dysfunction (n=29) groups, based on a study by David, et al.9 We used the NT-proBNP level of 5300 pg/mL as a standard for the determination of LV dysfunction.9 The comparisons of characteristics between both groups are shown in Table 2. A Mann-Whitney U test was then conducted to investigate the differences between the two groups. The results showed that the Kt/V, E/Ea ratio, pre-OH, and pre-copeptin values were all significantly different. Pre-copeptin increased in the patients with LV dysfunction more than in those with normal LV function (Fig. 2).
ROC curve analysis for the diagnosis of LV dysfunction showed that pre-copeptin and the E/Ea ratio had significant area under the curve values (Table 3). In addition, the cut-off value of pre-copeptin for the diagnosis of LV dysfunction was 125.48 pg/mL (sensitivity 0.7, specificity 0.8, positive predictive value 0.9, negative predictive value 0.6).
Previous literature reported that copeptin increased in dialysis patients with cardiovascular disease and predicted cardiovascular disease mortality.11 However, the general features of copeptin in hemodialysis patients were not investigated sufficiently, although copeptin was considered as a useful biomarker for dialysis patients with heart disease. In the case of vasopressin, several studies described a change in blood level during hemodialysis.12,13,14,15 They went on to report that vasopressin level was high before hemodialysis due to increased plasma osmolality and that vasopressin decreased if there was a fall in plasma osmolality, although body fluids were removed during hemodialysis. Furthermore, the studies indicated that the decreased vasopressin during hemodialysis was found to be associated with intradialytic hypotension and that hypertonic saline injection stimulated the release of vasopressin, which prevented intradialytic hypotension.16,17
Our results showed that the copeptin level in hemodialysis patients was higher than in healthy people. This result is similar to the case of vasopressin described by previous studies. However, our study also showed that copeptin had no significant correlation with plasma osmolality. Rather, copeptin had a significant positive correlation with pre-dialysis body fluid volumes. This result is confusing, as it is different from that of previous studies and body fluid amounts usually have a negative association with vasopressin levels. Our hypothesis is that hemodialysis patients may have various stimulating factors for vasopressin release irrelevant to plasma osmolality and body fluid volume, for example, a decrease in effective circulating volume, LV dysfunction, or nonspecific stress such as uremia. Schrier, et al.18 also indicated that vasopressin could be controlled by non-osmotic factors other than osmotic stimuli. We believe that hemodialysis patients with uncontrolled volume status usually have more of these non-osmotic stimulating factors for vasopressin release than patients with controlled volume status. Therefore, it is believed that copeptin demonstrates a positive association with body fluid volume and that the higher level of copeptin in hemodialysis patients is attributed to various non-osmotic factors as well as plasma osmolality. Additionally, our results showed that copeptin decreased together with plasma osmolality. According to previous research, vasopressin and plasma osmolality did not decrease during isolated ultrafiltration, though both decreased during conventional hemodialysis, which indicated that a decrease in vasopressin during hemodialysis was not due to the direct removal of vasopressin by hemodialysis.13,15,16 We believe that copeptin also changes in the same way as the vasopressin described previously in these studies.
Previous studies also indicated that copeptin was related to LV dysfunction in the patients who did not receive dialysis.8,19 Our study investigated whether copeptin could be related to LV dysfunction in hemodialysis patients. Based on a previous study, an NT-proBNP of 5300 pg/mL was set as a standard for the determination of LV dysfunction.9 When compared to the patients with normal LV function, the patients with LV dysfunction had higher pre-copeptin levels. Such a result is similar to the findings of copeptin from previous studies conducted on heart failure patients who did not receive dialysis. As a result, our study indicates that copeptin can be a useful marker for the diagnosis of LV dysfunction in hemodialysis patients. ROC curve analysis showed that pre-copeptin had a diagnostic value in the patients with LV dysfunction. The cut-off value was 125.48 pg/mL.
Collectively, our study indicates that copeptin increases in hemodialysis patients and is higher in hemodialysis patients with LV dysfunction. We believe that copeptin can be a useful marker for hemodialysis patients with LV dysfunction.
Figures and Tables
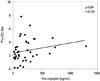 | Fig. 1Correlation between pre-dialysis copeptin (pre-copeptin) and pre-dialysis overhydration (pre-OH). OH, overhydration. |
 | Fig. 2Comparison of pre-dialysis copeptin (pre-copeptin) levels between patients with normal LV function and those with LV dysfunction. LV, left ventricular-. |
Table 1
Changes of Overhydration (OH) Index, Plasma Osmolality, and Copeptin during Hemodialysis

Table 2
Comparisons of Characteristics between Patients with Normal LV Function and Those with LV Dysfunction
References
1. Tentzeris I, Jarai R, Farhan S, Perkmann T, Schwarz MA, Jakl G, et al. Complementary role of copeptin and high-sensitivity troponin in predicting outcome in patients with stable chronic heart failure. Eur J Heart Fail. 2011; 13:726–733.

2. Maisel A, Xue Y, Shah K, Mueller C, Nowak R, Peacock WF, et al. Increased 90-day mortality in patients with acute heart failure with elevated copeptin: secondary results from the Biomarkers in Acute Heart Failure (BACH) study. Circ Heart Fail. 2011; 4:613–620.

3. Neuhold S, Huelsmann M, Strunk G, Stoiser B, Struck J, Morgenthaler NG, et al. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: prediction of death at different stages of the disease. J Am Coll Cardiol. 2008; 52:266–272.

4. Voors AA, von Haehling S, Anker SD, Hillege HL, Struck J, Hartmann O, et al. C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J. 2009; 30:1187–1194.

5. Alehagen U, Dahlström U, Rehfeld JF, Goetze JP. Association of copeptin and N-terminal proBNP concentrations with risk of cardiovascular death in older patients with symptoms of heart failure. JAMA. 2011; 305:2088–2095.

6. Potocki M, Reichlin T, Thalmann S, Zellweger C, Twerenbold R, Reiter M, et al. Diagnostic and prognostic impact of copeptin and high-sensitivity cardiac troponin T in patients with pre-existing coronary artery disease and suspected acute myocardial infarction. Heart. 2012; 98:558–565.

7. Morawiec B, Kawecki D. Copeptin: a new marker in cardiology. J Cardiovasc Med (Hagerstown). 2013; 14:19–25.
8. Kelly D, Squire IB, Khan SQ, Quinn P, Struck J, Morgenthaler NG, et al. C-terminal provasopressin (copeptin) is associated with left ventricular dysfunction, remodeling, and clinical heart failure in survivors of myocardial infarction. J Card Fail. 2008; 14:739–745.

9. David S, Kümpers P, Seidler V, Biertz F, Haller H, Fliser D. Diagnostic value of N-terminal pro-B-type natriuretic peptide (NT-proBNP) for left ventricular dysfunction in patients with chronic kidney disease stage 5 on haemodialysis. Nephrol Dial Transplant. 2008; 23:1370–1377.

10. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006; 52:112–119.

11. Fenske W, Wanner C, Allolio B, Drechsler C, Blouin K, Lilienthal J, et al. Copeptin levels associate with cardiovascular events in patients with ESRD and type 2 diabetes mellitus. J Am Soc Nephrol. 2011; 22:782–790.

12. Horký K, Srámková J, Lachmanová J, Tomásek R, Dvoráková J. Plasma concentration of antidiuretic hormone in patients with chronic renal insufficiency on maintenance dialysis. Horm Metab Res. 1979; 11:241–246.

13. Fasanella d'Amore T, Wauters JP, Waeber B, Nussberger J, Brunner HR. Response of plasma vasopressin to changes in extracellular volume and/or plasma osmolality in patients on maintenance hemodialysis. Clin Nephrol. 1985; 23:299–302.
14. Hegbrant J, Thysell H, Mårtensson L, Ekman R, Boberg U. Changes in plasma levels of vasoactive peptides during standard bicarbonate hemodialysis. Nephron. 1993; 63:303–308.

15. Os I, Nordby G, Lyngdal PT, Eide I. Plasma vasopressin, catecholamines and atrial natriuretic factor during hemodialysis and sequential ultrafiltration. Scand J Urol Nephrol. 1993; 27:93–99.

16. Thompson AM, Oliver JA. Endogenous and exogenous vasopressin during hemodialysis. Semin Dial. 2009; 22:472–475.

17. Ettema EM, Kuipers J, Groen H, Kema IP, Westerhuis R, de Jong PE, et al. Vasopressin release is enhanced by the Hemocontrol biofeedback system and could contribute to better haemodynamic stability during haemodialysis. Nephrol Dial Transplant. 2012; 27:3263–3270.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download