Abstract
Purpose
Although inconsistent, reports have shown fibrinogen levels to be associated with atherosclerosis. Accordingly, since cigarette smoking is associated with increased levels of fibrinogen and atherosclerosis, it may also affect the association between fibrinogen and atherosclerosis. We investigated the associations between fibrinogen and carotid intima-media thickness (IMT) according to smoking status in a Korean male population.
Materials and Methods
Plasma fibrinogen levels were measured in 277 men aged 40-87 years without a history of myocardial infarction or stroke. High-resolution B-mode ultrasonography was used to examine the common carotid arteries. IMT level was analyzed both as a continuous (IMT-max, maximum value; IMT-tpm, 3-point mean value) and categorical variable (higher IMT; presence of plaque). Serial linear and logistic regression models were employed to examine the association between fibrinogen and IMT according to smoking status.
Results
Fibrinogen levels were positively associated with IMT-max (standardized β=0.25, p=0.021) and IMT-tpm (standardized β=0.21, p=0.038), even after adjusting for age, body mass index, systolic blood pressure, fasting glucose, and total cholesterol to high-density lipoprotein cholesterol ratio in current smokers (n=75). No significant association between fibrinogen and IMT, however, was noted in former smokers (n=80) or nonsmokers (n=122). Adjusted odds ratios (95% confidence interval) for having plaque per one standard deviation higher fibrinogen level were 2.06 (1.09-3.89) for current smokers, 0.68 (0.43-1.10) for former smokers, and 1.06 (0.60-1.87) for nonsmokers.
As a hemostatic marker, plasma levels of fibrinogen are associated with the incidence of and mortality from cardiovascular disease.1,2 Fibrinogen levels have also been shown to be associated with carotid intima-media thickness (IMT),3,4,5,6 which is a marker of early atherosclerosis and a predictor of atherosclerotic cardiovascular disease.7 Some studies, however, have not found a significant association between fibrinogen level and carotid IMT.8,9,10 Cigarette smoking, an established risk factor for atherosclerosis, is also linked to increased fibrinogen levels.11,12,13 Possible mechanisms for the association between smoking and atherosclerosis include endothelial dysfunction, vessel wall injury, oxidative stress, platelet activation, and inflammation.14,15 Most of these mechanisms are also related to the association between fibrinogen level and atherosclerotic cardiovascular disease.16 In this context, some studies have proposed that smoking may influence the association between fibrinogen and cardiovascular risk.17,18,19 Therefore, to determine whether smoking status may explain the inconsistent relationship between fibrinogen levels and atherosclerosis reported in the literature, we aimed to assess the association between plasma fibrinogen level and carotid IMT according to smoking status in a middle-aged Korean population.
This study was designed as a cross-sectional analysis of a subsample cohort within an ongoing community-based cohort study. Between July and August 2010, a fibrinogen ancillary study was performed for 796 permanent residents (311 men, 485 women) of Ganghwa Island, Incheon, Korea. Because only a small number of female participants (2.08%) were current smokers, the analysis was limited to male participants. Among the 311 men with available fibrinogen levels, 34 individuals were excluded because no carotid IMT measurement was available (n=6) or there was a history of myocardial infarction or stroke (n=28). A total of 277 men were eligible for this study. Informed consent was obtained from each participant, and the study protocol was approved by the Institutional Review Board of Severance Hospital at Yonsei University College of Medicine.
A standardized questionnaire administered by trained interviewers was used to collect data on the participants' age, sex, medical history, and smoking status. Current smokers were defined as those who had smoked more than 100 cigarettes in their lifetime and reported that they were presently smoking. Former smokers comprised those who had smoked greater than 100 cigarettes in their lifetime but did not smoke recently. Nonsmokers included those who had smoked fewer than 100 cigarettes in their lifetime. The height and weight of each participant were measured and body mass index was calculated as weight divided by height squared (kg/m2). Participants were seated for at least five minutes before blood pressure measurement, and two measurements at least 5-minute intervals were obtained by an automatic sphygmomanometer (Dinamap 1846 SX/P; GE Healthcare, Waukesha, WI, USA). If the two measurements differed by ≥10 mm Hg, for either systolic or diastolic blood pressure, an extra measurement was taken after 5 minutes; the last two measurements were averaged for analysis. Venous blood samples were collected after a minimum 8-hour fast. Enzymatic methods (ADVIA 1800; Siemens, New York, USA) were used to measure glucose, alanine transaminase, aspartate transaminase, total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels. Friedewald, et al.'s20 formula was used to calculate the level of low-density lipoprotein cholesterol. Turbidimetric immunoassay (ADVIA 1800; Siemens, New York, USA) was used to measure high-sensitivity C-reactive protein. White blood cell counts were measured by the impedance method with an automatic analyzer (ADIVA120; Siemens, New York, USA). Fibrin d-dimer was measured via an enzyme linked fluorescent assay (Mini vidas; BioMerieux SA, Lyon, France). Plasma fibrinogen level was measured by turbidimetric immunoassay (Sta-R; Diagnostica Stago, Asnieres, France). Intra- and inter-assay coefficients for fibrinogen were less than 5%.
Right and left common carotid arteries were assessed with high-resolution B-mode ultrasonography (SSAD-3500SV; Aloka, Tokyo, Japan) and a 7.5 MHz linear array transducer. Each participant was placed in the supine position with the head turned at a 45° angle contralateral to the scanning side. Carotid IMT was measured at the end-diastolic phase along the vertical distance between the leading edge of the first and second echogenic lines of the far wall of the common carotid artery (IntimaScope; MediaCross, Tokyo, Japan). IMT was measured at three points (IMT-tpm) along the roughly 20-mm region: both ends and the midpoint of the region. The maximal IMT value was defined as the greatest IMT value along the region. For statistical analysis, IMT-tpm was defined as the average value of the right and left sides of the common carotid artery; IMT-max comprised the highest value for both sides; and higher IMT was operationally defined as the upper 25% of IMT-tpm measurements. Carotid artery plaque was defined as an IMT-max>1.0 mm or an area of focal wall thickening that was 100% or greater than the IMT of neighboring sites.21,22
Clinical characteristics were compared according to smoking status. Differences were assessed with analysis of variance, the Kruskal-Wallis test, or the chi-square test. Fisher's exact test was applied if there were less than five observations per cell. Linear regression analysis was performed to assess the relationship between fibrinogen and continuous IMT values. Logistic regression analysis was performed to estimate the odds ratio for atherosclerosis (higher IMT or presence of carotid plaque) per one standard deviation (SD) increase in fibrinogen level. All regression analyses were performed before and after adjusting for age, body mass index, systolic blood pressure, fasting glucose, and ratio of total cholesterol to HDL cholesterol. The ratio of total to HDL cholesterol was used as a marker of dyslipidemia, because it reflects the balance between atherogenic and antiatherogenic lipoproteins and is known to be associated with the risk of atherosclerotic cardiovascular disease.23 Moreover, the ratio explained IMT variation more than the other markers of dyslipidemia in our current analysis, as well as in our previous reports.24,25 All statistical tests were performed with SAS version 9.2 (SAS Inc., Cary, NC, USA). All analyses were two-sided and p-values less than 0.05 were regarded as statistically significant.
The mean age of study participants (n=277) was 60.4 years, and the mean fibrinogen level was 2.9 g/L. Compared with current smokers and nonsmokers, former smokers were older and had higher fibrinogen levels (Table 1). However, there was no significant difference in blood pressure, cholesterol, fasting glucose, or C-reactive protein levels according to smoking status. Former smokers had higher IMT-tpm levels than nonsmokers and current smokers, although the difference was of borderline statistical significance (p=0.084). There were no significant differences in IMT-max or the presence of carotid plaque between nonsmokers, former smokers, and current smokers.
Fig. 1 displays the relationship between fibrinogen level and IMT-tpm according to smoking status. In contrast with nonsmokers and former smokers, as fibrinogen levels increased, a significant increase in IMT-tpm was noted in current smokers (p=0.029). Fig. 2 depicts the relationship between fibrinogen levels and IMT-max according to the smoking status. Similarly to the findings for IMT-tpm, fibrinogen levels showed a positive association with IMT-max in current smokers (p=0.012), but not in former smokers or nonsmokers.
Table 2 shows the relationship between fibrinogen level and continuous measures of IMT, before and after adjusting for age, body mass index, blood pressure, fasting glucose, and total to HDL cholesterol ratio. In nonsmokers and former smokers, fibrinogen level was not linearly associated with IMT-tpm or IMT-max. This null finding was consistent before and adjustment for potential confounders. However, in current smokers, fibrinogen level was positively associated with IMT-tpm (p=0.029) and IMT-max (p=0.012). Even after adjustment for potential confounders, fibrinogen level was significantly associated with both IMT-tpm (p=0.038) and IMT-max (p=0.021). In current smokers, fibrinogen level explained the variations in IMT-tpm and IMT-max by approximately 6% and 8%, respectively.
Table 3 lists the odds ratios for carotid atherosclerosis (defined as higher IMT or presence of carotid plaque) for one SD increase in fibrinogen level for nonsmokers, former smokers, and current smokers. Fibrinogen level was significantly associated with the presence of carotid atherosclerosis only in current smokers. This result was consistent before and after adjustment for potential confounders.
In this community-based study, the association between fibrinogen level and atherosclerosis differed according to smoking status. Among current smokers, there was a significant positive association between fibrinogen level and carotid IMT, even after adjusting for potential confounders. This association was not present for nonsmokers or former smokers. Some studies have suggested a positive association between fibrinogen and atherosclerosis.3,4,5 However, this positive association has been inconsistently demonstrated.8,9,10 Our results suggest that cigarette smoking might be an effect modifier on the association between fibrinogen and atherosclerosis. This effect modification by cigarette smoking may at least partially explain the inconsistent reports of an association between fibrinogen and atherosclerosis.
Studies have proposed that an association between fibrinogen and atherosclerotic cardiovascular disease might be affected, at least in part, by smoking.26,27 The effect of smoking on the relationship between fibrinogen level and atherosclerosis can be explained in several ways: first, oxidative stress from cigarette smoking can modulate the function of fibrinogen and fibrin architecture. Previous studies have suggested that smoking can contribute to increasing clot strength related to the kinetics of clot formation and the rapidity of fibrin build up. After exposure to cigarette smoke, fibrin clots exhibit thinner fibrin fibers, increased fibrin fiber density, and more uniform fiber distribution, compared with pre-smoking and non-smoking samples.28 These alterations in fibrin architecture may contribute to premature coronary atherothrombosis.29 A fibrin clot with a high fiber density would cause delayed clot lysis and thrombotic disease.30 Second, smoking can affect fibrinolysis via alteration of plasminogen activator inhibitor type 1 (PAI-1).31 Smokers have higher levels of PAI-1 than nonsmokers.32 PAI-1 inhibits the fibrinolytic pathway by binding both urinary-type plasminogen activator and tissue plasminogen activator, which are activators of plasminogen for fibrin clot lysis. It has been suggested that high plasma PAI-1 level in smokers can contribute to the formation of thrombi.33 In this context, alteration of PAI-1 levels due to smoking may be related to carotid atherosclerosis. Finally, serum fibrinogen levels might be an indicator of chronic exposure to cigarette smoke and/or individual susceptibility to cigarette smoke. Studies have shown that cigarette smoking can increase the fibrinogen levels and arterial wall thickness.11,12,34
Interestingly, in the present study, although fibrinogen levels were significantly higher in former smokers than in current or nonsmokers, there was no significant association between fibrinogen and IMT among former smokers. The higher fibrinogen concentrations of former smokers were largely explained by age distribution. It is known that ageing is positively associated with fibrinogen level and other proinflammatory biomarkers.35 In our dataset, former smokers were older than current smokers or nonsmokers, and fibrinogen levels were not statistically different according to smoking status after adjusting for age. Not only current smoking but also past smoking can interact with fibrinogen and contribute to the development of carotid atherosclerosis, because atherosclerosis is a chronic progressive condition. However, we could not observe an association between fibrinogen and atherosclerosis among former smokers. The former smokers in our study were not entirely homogeneous, as some of them might have smoked cigarettes for a long period and only recently quit, while others may not have smoked for a long time. It has been reported that cardiovascular risk gradually decreases after smoking cessation and that fibrinogen and other metabolic risk profiles among quitters differ by the length of non-smoking period.36,37,38 Notwithstanding, we were unable to further classify former smokers according to smoking and non-smoking periods. Since the association between fibrinogen and carotid atherosclerosis was heterogeneous between former smokers and current smokers, we analyzed former smokers and current smokers separately rather than pooling the two smoker groups. However, as a further analysis, we developed multiple logistic regression models to assess the effects of fibrinogen on the risk of higher IMT and presence of plaque in the pooled smokers. The associations between fibrinogen and carotid atherosclerosis in the pooled smokers were much weaker than in those among current smokers: the adjusted odds ratio per 1 SD increase in fibrinogen was 1.42 (95% confidence interval, 0.98 to 2.07) for higher IMT and 1.19 (95% confidence interval, 0.85 to 1.68) for carotid plaque.
Our study has several limitations that warrant consideration. First, we could not observe time dependent changes in fibrinogen level or carotid IMT because of the cross-sectional study design. Thus, we could not evaluate causal relationships between cigarette smoking, fibrinogen level, and development of atherosclerosis. Second, we could not rule out the possibility that our findings were due to chance, partly because the number of current smokers was relatively small. Third, we could not assess the effects of smoking cessation and non-smoking period on the association between fibrinogen and atherosclerosis due to the small sample size. Further studies are required to address this issue. Finally, our results cannot be applied to other populations because the analysis was limited to a male population of a single rural community in Korea.
In conclusion, our findings suggest that cigarette smoking may modify the association between fibrinogen and carotid atherosclerosis. The study findings were consistent for continuous and categorical measures of atherosclerosis, and after adjustment for the potential confounding effects of age, obesity, blood pressure, fasting glucose, and cholesterol. Further studies of different populations are required to confirm the noted interactions between cigarette smoking, fibrinogen level, and atherosclerosis.
Figures and Tables
Fig. 1
Relationship between fibrinogen and IMT-tpm according to smoking status. (A) Nonsmokers. IMT-tpm=0.7056+0.0046×fibrinogen, p=0.8802. (B) Former smokers. IMT-tpm=0.7023+0.021×fibrinogen, p=0.3592. (C) Current smokers. IMT-tpm=0.5077+0.0734×fibrinogen, p=0.0291. IMT, intima-media thickness; IMT-tpm, mean value of IMT at three points.

Fig. 2
Relationship between fibrinogen and IMT-max according to smoking status. (A) Nonsmokers. IMT-max=0.9395+0.0115×fibrinogen, p=0.8594. (B) Former smokers. IMT-max=0.9893+0.0183×fibrinogen, p=0.7532. (C) Current smokers. IMT-max=0.5245+0.1596×fibrinogen, p=0.0119. IMT, intima-media thickness; IMT-max, maximum IMT value.

Table 1
Characteristics of the Participants According to Smoking Status
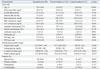
Table 2
Association between Fibrinogen Level and Continuous Measure of IMT According to Smoking Status

Table 3
Association between Fibrinogen Level and Categorical Measure of IMT According to Smoking Status

ACKNOWLEDGEMENTS
This work was supported by the Global Research Network (GRN) Program, National Research Foundation of Korea (NRF), Ministry of Education, Science and Technology (Grant No. 220-2009-1-E00023) and by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. HI13C0715).
References
1. Fibrinogen Studies Collaboration. Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005; 294:1799–1809.
2. de Moerloose P, Boehlen F, Neerman-Arbez M. Fibrinogen and the risk of thrombosis. Semin Thromb Hemost. 2010; 36:7–17.

3. Green D, Foiles N, Chan C, Schreiner PJ, Liu K. Elevated fibrinogen levels and subsequent subclinical atherosclerosis: the CARDIA Study. Atherosclerosis. 2009; 202:623–631.

4. Páramo JA, Beloqui O, Roncal C, Benito A, Orbe J. Validation of plasma fibrinogen as a marker of carotid atherosclerosis in subjects free of clinical cardiovascular disease. Haematologica. 2004; 89:1226–1231.
5. Grebe MT, Luu B, Sedding D, Heidt MC, Kemkes-Matthes B, Schaefer CA, et al. Fibrinogen promotes early atherosclerotic changes of the carotid artery in young, healthy adults. J Atheroscler Thromb. 2010; 17:1003–1008.

6. Green D, Chan C, Kang J, Liu K, Schreiner P, Jenny NS, et al. Longitudinal assessment of fibrinogen in relation to subclinical cardiovascular disease: the CARDIA study. J Thromb Haemost. 2010; 8:489–495.

7. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007; 115:459–467.

8. Hayashi S. Significance of plasma D-dimer in relation to the severity of atherosclerosis among patients evaluated by non-invasive indices of cardio-ankle vascular index and carotid intima-media thickness. Int J Hematol. 2010; 92:76–82.

9. Sekikawa A, Ueshima H, Kadowaki T, El-Saed A, Okamura T, Takamiya T, et al. Less subclinical atherosclerosis in Japanese men in Japan than in White men in the United States in the post-World War II birth cohort. Am J Epidemiol. 2007; 165:617–624.

10. Choi H, Cho DH, Shin HH, Park JB. Association of high sensitivity C-reactive protein with coronary heart disease prediction, but not with carotid atherosclerosis, in patients with hypertension. Circ J. 2004; 68:297–303.

11. Kannel WB, D'Agostino RB, Belanger AJ. Fibrinogen, cigarette smoking, and risk of cardiovascular disease: insights from the Framingham Study. Am Heart J. 1987; 113:1006–1010.

12. Meade TW, Imeson J, Stirling Y. Effects of changes in smoking and other characteristics on clotting factors and the risk of ischaemic heart disease. Lancet. 1987; 2:986–988.

13. Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003; 138:891–897.

14. Leone A. Smoking, haemostatic factors, and cardiovascular risk. Curr Pharm Des. 2007; 13:1661–1667.

15. Unverdorben M, von Holt K, Winkelmann BR. Smoking and atherosclerotic cardiovascular disease: part II: role of cigarette smoking in cardiovascular disease development. Biomark Med. 2009; 3:617–653.

16. Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011; 364:1746–1760.

17. Ernst E. Fibrinogen as a cardiovascular risk factor--interrelationship with infections and inflammation. Eur Heart J. 1993; 14:Suppl K. 82–87.
18. Woodward M, Lowe GD, Rumley A, Tunstall-Pedoe H. Fibrinogen as a risk factor for coronary heart disease and mortality in middle-aged men and women. The Scottish Heart Health Study. Eur Heart J. 1998; 19:55–62.

19. Kannel WB. Overview of hemostatic factors involved in atherosclerotic cardiovascular disease. Lipids. 2005; 40:1215–1220.

20. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502.

21. Yanase T, Nasu S, Mukuta Y, Shimizu Y, Nishihara T, Okabe T, et al. Evaluation of a new carotid intima-media thickness measurement by B-mode ultrasonography using an innovative measurement software, intimascope. Am J Hypertens. 2006; 19:1206–1212.

22. Lemne C, Jogestrand T, de Faire U. Carotid intima-media thickness and plaque in borderline hypertension. Stroke. 1995; 26:34–39.

23. Castelli WP, Abbott RD, McNamara PM. Summary estimates of cholesterol used to predict coronary heart disease. Circulation. 1983; 67:730–734.

24. Chang HS, Kim HC, Ahn SV, Hur NW, Suh I. Impact of multiple cardiovascular risk factors on the carotid intima-media thickness in young adults: the Kangwha Study. J Prev Med Public Health. 2007; 40:411–417.

25. Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima-media thickness according to the presence of metabolic syndrome. Atherosclerosis. 2009; 204:521–525.

26. Fowkes FG, Lee AJ, Lowe GD, Riemersma RA, Housley E. Inter-relationships of plasma fibrinogen, low-density lipoprotein cholesterol, cigarette smoking and the prevalence of cardiovascular disease. J Cardiovasc Risk. 1996; 3:307–311.

27. Tuut M, Hense HW. Smoking, other risk factors and fibrinogen levels. evidence of effect modification. Ann Epidemiol. 2001; 11:232–238.

28. Barua RS, Sy F, Srikanth S, Huang G, Javed U, Buhari C, et al. Effects of cigarette smoke exposure on clot dynamics and fibrin structure: an ex vivo investigation. Arterioscler Thromb Vasc Biol. 2010; 30:75–79.

29. Collet JP, Allali Y, Lesty C, Tanguy ML, Silvain J, Ankri A, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006; 26:2567–2573.

30. Lord ST. Molecular mechanisms affecting fibrin structure and stability. Arterioscler Thromb Vasc Biol. 2011; 31:494–499.
31. Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007; 131:1557–1566.

32. Yarnell JW, Sweetnam PM, Rumley A, Lowe GD. Lifestyle and hemostatic risk factors for ischemic heart disease: the Caerphilly Study. Arterioscler Thromb Vasc Biol. 2000; 20:271–279.
33. Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000; 342:1792–1801.

34. Howard G, Burke GL, Szklo M, Tell GS, Eckfeldt J, Evans G, et al. Active and passive smoking are associated with increased carotid wall thickness. The Atherosclerosis Risk in Communities Study. Arch Intern Med. 1994; 154:1277–1282.

35. Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood. 2005; 105:2294–2299.

36. Kramer A, Jansen AC, van Aalst-Cohen ES, Tanck MW, Kastelein JJ, Zwinderman AH. Relative risk for cardiovascular atherosclerotic events after smoking cessation: 6-9 years excess risk in individuals with familial hypercholesterolemia. BMC Public Health. 2006; 6:262.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download