Abstract
Purpose
To investigate the effect of antifreeze protein (AFP) supplementation on ovarian vitrification and transplantation.
Materials and Methods
In this experimental study, we researched a total of 182 ovaries from 4-week-old ICR mice. The equilibration solution included 20% ethylene glycol (EG), and the vitrification solution included 40% EG, 18% Ficoll, and 0.3 M sucrose. Intact ovaries were first suspended in 1 mL of equilibration solution for 10 min, and then mixed with 0.5 mL of vitrification solution for 5 min. Ovaries were randomly assigned to 3 groups and 0, 5, or 20 mg/mL of type III AFP was added into the vitrification solution (control, AFP5, and AFP20 groups, respectively). The vitrified ovaries were evaluated after warming and 2 weeks after autotransplantation. The main outcome measurements are follicular morphology and apoptosis assessed by histology and the TUNEL assay.
Results
A significantly higher intact follicle ratio was shown in the AFP treated groups (control, 28.9%; AFP5, 42.3%; and AFP20, 44.7%). The rate of apoptotic follicles was significantly lower in the AFP treated groups (control, 26.6%; AFP5, 18.7%; and AFP20, 12.6%). After transplantation of the vitrified-warmed ovaries, a significantly higher intact follicle ratio was shown in the AFP20 group. The rate of apoptotic follicles was similar among the groups.
Ovarian cryopreservation and transplantation are one of the most promising options for fertility preservation of female cancer survivors because it has several advantages compared with other options. First, since ovarian stimulation is not necessary for ovarian cryopreservation, it only minimally interferes with the treatment plan. In addition, a male partner is not required, and thus, this procedure is an option for single women and prepubertal girls. Several reports have indicated that endocrine function and fertility are restored after transplantation of frozen-thawed ovarian tissue. Moreover, successful pregnancies have also been reported by several authors.1 Currently, more than 20 live births have been reported in humans after transplantation of cryopreserved ovarian tissue.2
Despite these encouraging results, some concerns still limit the application of this procedure. Damage to ovarian tissue during cryopreservation thawing and transplantation is associated with 2 key factors: 1) cryodamage that occurs during cryopreservation thawing and 2) the ischemic injury that occurs during revascularization of the transplanted tissue from the surrounding vessels. One of the strategies for overcoming these obstacles is to supplement the patient with protective agents such as antioxidants,3,4,5,6 anti-apoptotic agents,7,8 or angiogenic factors9,10 during cryopreservation and/or transplantation. Some studies showed beneficial effects, but others couldn't. And there were controversies among the reports that have studied the same agent.
Antifreeze proteins (AFPs) are a class of polypeptides produced by animals such as antarctic fish; these proteins allow their survival in subzero environments.11 Several protective mechanisms such as a decrease in freezing temperature, inhibition of recrystallization during thawing, and protection of plasma membrane at low temperatures have been proposed.12 Recently, studies on the protective effects of AFP for cryopreservation of animal cells and organs have been reported.13,14,15,16 However, no study has been performed on the use of AFP for ovarian tissue cryopreservation. The aims of this study were to investigate the effect of AFP supplementation during ovarian tissue vitrification procedures and to development an improved technique of ovarian cryopreservation for fertility preservation.
The experimental procedures performed were similar to those described previously8 and are presented in Fig. 1. The animals in this study were cared for and used in accordance with the institutional guidelines established by the Animal Care and Use Committee (IACUC) of Seoul National University of Bundang Hospital. ICR mice (Orient Co., Seoul, Korea) were maintained under 12-h light: 12-h dark cycle at 23℃ and fed ad libitum. After 1 week of adaptation, 4-week-old mice were killed by cervical dislocation, and both the ovaries were resected. Ovaries were vitrified using a two-step method involving exposure to equilibrium and vitrification solutions.17 Equilibration solution had 20% ethylene glycol (EG; Sigma Chemical Co., St. Louis, MO, USA); and vitrification solution was composed of 40% EG, 18% Ficoll (Sigma), and 0.3 M sucrose (Sigma). All solutions were based on Dulbecco's phosphate buffered saline (DPBS; Gibco BRL, Grand Island, NY, USA) containing 20% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA).
Intact ovaries were first suspended in 1 mL of equilibration solution for 10 min and then mixed with 0.5 mL of vitrification solution for 5 min. To investigate the effect of AFP, 0, 5, or 20 mg/mL of AFP type III (AFP III, A/F Protein Inc., Waltham, MA, USA) was added to the vitrification solution. Because there was no previous study using AFP on mouse ovarian cryopreservation, the doses of AFP for treated groups were determined on the basis of the previous studies on pig oocyte vitrification and rat heart transplantation.15,18 After exposure to equilibrium and vitrification solutions, ovaries were immediately transferred to 1.2-mL cryotubes (Nunc; Fisher Bioblock Scientific, Illkirch, France) and directly plunged into liquid nitrogen. After 2 weeks, the tissues were warmed by immersing the vials rapidly in a water bath at 37℃ and suspended serially in 0.5 M sucrose in DPBS containing 20% FBS for 5 min, 0.25 M for 5 min, and 0 M for 10 min.
All the ovarian samples were fixed in 10% buffered formalin and then embedded in paraffin block. For routine histologic examination, paraffin embedded ovarian sections were cut (thickness, 5 µm). After deparaffinization and rehydration, the sections were stained with hematoxylin and eosin. The numbers of each type of follicle were counted in the entire cut ovarian surface (×400). All follicles found in 1 section of each ovary were scored. To avoid miscounting, the follicles were analyzed in only one section per ovary when they contained oocytes. Each type of follicle was categorized according to the following classification.19
The integrity of each follicle was evaluated using the following criteria:20
Paraffin-embedded ovarian sections were cut (thickness, 5 µm) and assessed for apoptosis using a commercial TUNEL assay kit (In Situ Cell Death Detection kit, Fluorescein, Roche Applied Science, Penzberg, Germany). After deparaffinization and rehydration, sections were rinsed in phosphate buffered saline (pH 7.2) and digested using proteinase K (20 µg/mL, 37℃, 30 min) in 10 mM Tris-HCl buffer. After rinsing in DPBS, the entire specimens were incubated with 50 µL of TUNEL reaction mixture at 37℃ for 60 min in a humidified chamber in the dark, followed by rinsing with DPBS. Positive control slides were prepared by treating 1500 U/mL DNase I (Roche Applied Science) in 50 mM Tris-HCl (pH 7.5, including 1 mg/mL bovine serum albumin) for 10 min at room temperature to induce DNA strand breaks, prior to labeling procedures. Some ovarian tissue specimens were used as negative controls by substituting TdT with distilled water in the reaction mixture following the protocol.
The slides were then covered with DAPI (Vector Laboratories, Burlingame, CA, USA) to counterstain DNA. TUNEL-stained and DAPI counterstained slides were examined under an inverted fluorescence microscope (Carl Zeiss, Oberkochen, Germany). Green fluorescence was visualized in TUNEL-positive cells at an excitation wavelength in the range of 450-500 nm and detection in the range of 515-565 nm. DAPI reached excitation at approximately 360 nm and emitted at approximately 460 nm when bound to DNA, producing blue fluorescence in all nuclei. Only follicles with a visible nucleus were counted regardless of their types. Follicles were considered apoptotic if >30% of the follicular cells were TUNEL stained.
Two weeks after vitrification, cryopreserved ovaries were warmed and immediately autografted into the dorsal subcutaneous space of the flank. Two weeks later, the transplanted ovarian tissues were retrieved, and their gross morphology was examined. Using only normal-appearing intact ovaries, the follicular normality and apoptosis were assessed as described above, and immunohistochemical analysis was performed.
The proportions of follicle stages and normality were calculated in each group. Statistical analysis was performed using analysis of variance, Student's t-test or Kruskal Wallis test for continuous variables, whereas chi-square or Fisher's exact tests were used for categorical variables, as appropriate. Results were considered statistically significant for p-values<0.05. The statistical software package SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
Morphological analysis after vitrification and warming showed a significantly higher intact follicle (G1) ratio in the AFP treated groups (controls, 28.9%; 5 mg/mL AFP treated group, 42.3%; and 20 mg/mL AFP treated group, 44.7%) (Fig. 2, Table 1). When follicle types were considered separately, AFP also showed protective effects. In all follicle types, the AFP treated groups showed a high proportion of intact (G1) follicles, although the values did not reach statistical significance. The rate of apoptotic follicles (TUNEL positive) was significantly lower in the AFP treated groups (control, 26.6%; 5 mg/mL AFP treated group, 18.7%; and AFP 20 mg/mL treated group, 12.6%), and the rate was decreased more with higher dose of AFP supplementation (Figs. 3 and 4). Intact primordial follicle ratio of 5 mg/mL AFP treated group was higher than control and 20 mg/mL AFP treated group (control, 22.5%; 5 mg/mL AFP treated group, 31.5%; and AFP 20 mg/mL treated group, 24.6%), but this difference was not statistically significant.
After transplantation of the vitrified-warmed ovaries, the percentage of grossly normal ovary seemed to increase as the AFP dose increased, but the difference was not statistically significant (control, 70.3%; 5 mg/mL AFP treated group, 78.9%; and 20 mg/mL AFP treated group, 88.0%). Morphological analysis of grossly normal ovaries showed a significantly higher intact follicle (G1) ratio in the 20 mg/mL AFP treated group than the control and the 5 mg/mL AFP treated groups (Fig. 2, Table 2). The rate of apoptotic follicles was similar among the groups (control, 8.3%; 5 mg/mL AFP treated group, 7.8%; and 20 mg/mL AFP treated group, 7.5%) (Fig. 5). Intact primordial follicle ratio of both AFP treated groups were higher than control group (control, 35.9%; 5 mg/mL AFP treated group, 44.0%; and AFP 20 mg/mL treated group, 43.7%), but this difference was not statistically significant.
In the present study, the AFP treated group showed a significantly higher intact follicle ratio. In addition, the rate of apoptotic follicles was significantly lower in the AFP treated groups than the controls. These positive effects of AFP supplementation were also evident after transplantation, but statistically significant difference was noted only in the higher dose (20 mg/mL) AFP group. These results demonstrate that AFP supplementation during vitrification improves the survival of ovarian tissue during cryopreservation and after transplantation.
AFPs are a class of polypeptides produced by animals such as antarctic fish; these proteins allow their survival in subzero environments.11 Five groups of these proteins exist, one of which is glycosylated (antifreeze glycoproteins, AFGPs). Structural characterization and properties of AFPs and AFGPs and the molecular mechanisms involved in inhibiting ice growth are still not completely clear. Suggested cryoprotective mechanisms include 1) a decrease in freezing temperature or thermal hysteresis and the inhibition of the normal growth habit of ice,21,22,23 2) inhibition of recrystallization during warming,24,25 and 3) protection of the plasma membrane.26
Previous studies have demonstrated that AFPs are effective cryoprotectants for oocytes, embryos, and spermatozoa. The vitrification of immature oocytes and two-cell-stage embryos of mice or pigs treated with AFGPs at 40 mg/mL showed improvements in morphological integrity, suggesting that these proteins can inhibit ice formation and stabilize the plasma membrane.26 O'Neil, et al.27 found significantly enhanced rates of fertilization after mature mouse oocyte vitrification with 6 mol/L Me2SO (dimethyl sulfoxide) plus 1 mg/mL AFGP. Supplementation with AFPs has also shown beneficial effects in bovine, ram, and chimpanzee spermatozoa.14,28,29 However, controversial results have also been reported. In these studies, AFPs did not show any beneficial effects on the survival of various cells, such as bovine blastocysts, oyster oocytes, and equine embryos.12 More recent studies with mature and immature mouse oocytes have demonstrated that supplementation of AFP III in the vitrification medium protects oocytes from chilling injury.13,30
There have been few studies which investigated the effect of AFP on ovarian tissue cryopreservation, but the results of many other studies on cryopreservation of other reproductive cells and embryos were in line with the present study. The mechanism of cryoprotective effect during vitrification in the present study is not clear. However, protective mechanism suggested by previous studies may be applicable to ovarian tissue cryopreservation. Jo, et al.13 suggested that one protective mechanism in mouse oocytes involved preservation of structural and functional integrity associated with the maintenance and recovery of spindle reassembly during vitrification and warming, and Bagis, et al.31 demonstrated that the litter size of mice that were transplanted vitrified ovaries from AFP III transgenic mice was not different from the control group, whereas the litter size of mice that were transplanted vitrified ovaries from non-transgenic mouse was significantly decreased. These results suggest that AFP III has beneficial effect for ovarian vitrification and transplantation. A potential protective mechanism may involve the interaction of AFP with function and structure of the cell membrane. In the present study, AFP III was added only in the vitrification solution, and therefore, the beneficial effect of AFP did not directly affect post-transplantation ischemic damage. However, the protective effect during vitrification and warming was enough to improve outcomes after transplantation. It is obvious that AFP has protective effects during ovarian vitrification and warming. Further study is necessary to elucidate the exact mechanism underlying this protective effect.
Several studies reporting a detrimental effect of AFP showed that high AFP concentrations were associated with a destructive effect on cells and tissues. In those studies, AFPs at relatively low concentration enhanced the survival rate of red blood cells, whereas at high concentrations these proteins reduced survival rates.24,25 A recent study involving immature mouse oocytes showed that high doses of AFP have a harmful effect on oocyte survival.30 In the present study, a high dose of AFP (20 mg/mL) demonstrated better results than lower dose (5 mg/mL). Further study is necessary to confirm whether AFP at higher doses is harmful, and also to determine a more optimal dose of AFP for ovarian tissue cryopreservation.
In a review on the effect and mechanisms of AFPs, Wang12 explained that AFPs have both protective and destructive actions depending on many relevant factors such as composition and concentration of cryoprotectant, type and concentration of AFPs, cooling and warming rate, and cell surface features. In the present study, the composition of equilibrium and vitrification solutions, exposure time to cryoprotectants, and the vitrification method are well-established protocols after long-term clinical use in embryo vitrification and confirmed by our previous study for optimization of vitrification method using human ovarian tissue.17 Thus, relevant factors influencing AFP effect have already been optimized to show protective rather than destructive effects.
In conclusion, the present results suggest that supplementation of AFP in the vitrification solution has beneficial effects on the survival of ovarian tissue during cryopreservation and transplantation. AFP may represent promising supplementary agents for reducing cryodamage during vitrification of ovarian tissue. Further studies with human ovarian tissue and evaluating toxicity are necessary for the application of this technique in clinical practice.
Figures and Tables
 | Fig. 1Schematic representation of the experimental design. n, number of ovaries in each step; AFP5, antifreeze protein, 5 mg/mL treated group; AFP20, antifreeze protein 20 mg/mL treated group. |
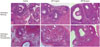 | Fig. 2Histology of ovaries in control, antifreeze protein (AFP) 5 mg/mL treated group, and AFP 20 mg/mL treated group after vitrification-warming and autotransplantation (×200). |
 | Fig. 3Fluorescent TUNEL staining for vitrified-warmed ovaries of control group, antifreeze protein (AFP) 5 mg/mL treated group, AFP 20 mg/mL treated group, positive and negative control (×100, DAPI-counterstained). DAPI, 4',6-Diamidino-2-phenylindole. |
 | Fig. 4Ratios of morphologically intact (grade I) follicle and apoptotic follicle according to supplementation of antifreeze protein (AFP) in vitrification solution after warming. |
 | Fig. 5Ratios of morphologically intact (grade I) follicle and apoptotic follicle according to supplementation of antifreeze protein (AFP) in vitrification solution after autotransplantation of vitrified-warmed ovary. |
Table 1
Proportions of Follicles with Good Morphology (Grade I) and Apoptotic Follicles According to Supplementation of Antifreeze Protein III in Vitrification Solution after Warming

Table 2
Proportions of Follicles with Good Morphology (Grade I) and Apoptotic Follicles According to Supplementation of Antifreeze Protein III in Vitrification Solution after Autotransplantation of Vitrified-Warmed Ovary

ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No.: HI12C0055).
References
1. Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009; 15:649–665.

2. Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012; 98:720–725.

3. Weissman A, Gotlieb L, Colgan T, Jurisicova A, Greenblatt EM, Casper RF. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol Reprod. 1999; 60:1462–1467.

4. Sağsöz N, Kisa U, Apan A. Ischaemia-reperfusion injury of rat ovary and the effects of vitamin C, mannitol and verapamil. Hum Reprod. 2002; 17:2972–2976.

5. Sapmaz E, Ayar A, Celik H, Sapmaz T, Kilic N, Yasar MA. Effects of melatonin and oxytetracycline in autologous intraperitoneal ovary transplantation in rats. Neuro Endocrinol Lett. 2003; 24:350–354.
6. Karaca M, Odabasoglu F, Kumtepe Y, Albayrak A, Cadirci E, Keles ON. Protective effects of erythropoietin on ischemia/reperfusion injury of rat ovary. Eur J Obstet Gynecol Reprod Biol. 2009; 144:157–162.

7. Onions VJ, Mitchell MR, Campbell BK, Webb R. Ovarian tissue viability following whole ovine ovary cryopreservation: assessing the effects of sphingosine-1-phosphate inclusion. Hum Reprod. 2008; 23:606–618.

8. Jee BC, Lee JR, Youm H, Suh CS, Kim SH, Moon SY. Effect of sphingosine-1-phosphate supplementation on follicular integrity of vitrified-warmed mouse ovarian grafts. Eur J Obstet Gynecol Reprod Biol. 2010; 152:176–180.

9. Schnorr J, Oehninger S, Toner J, Hsiu J, Lanzendorf S, Williams R, et al. Functional studies of subcutaneous ovarian transplants in non-human primates: steroidogenesis, endometrial development, ovulation, menstrual patterns and gamete morphology. Hum Reprod. 2002; 17:612–619.

10. Suzuki H, Ishijima T, Maruyama S, Yanagimoto Ueta Y, Abe Y, Saitoh H. Beneficial effect of desialylated erythropoietin administration on the frozen-thawed canine ovarian xenotransplantation. J Assist Reprod Genet. 2008; 25:571–575.

11. Yeh Y, Feeney RE. Antifreeze Proteins: Structures and Mechanisms of Function. Chem Rev. 1996; 96:601–618.

12. Wang JH. A comprehensive evaluation of the effects and mechanisms of antifreeze proteins during low-temperature preservation. Cryobiology. 2000; 41:1–9.

13. Jo JW, Jee BC, Lee JR, Suh CS. Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence. Fertil Steril. 2011; 96:1239–1245.

14. Prathalingam NS, Holt WV, Revell SG, Mirczuk S, Fleck RA, Watson PF. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology. 2006; 66:1894–1900.

15. Amir G, Rubinsky B, Horowitz L, Miller L, Leor J, Kassif Y, et al. Prolonged 24-hour subzero preservation of heterotopically transplanted rat hearts using antifreeze proteins derived from arctic fish. Ann Thorac Surg. 2004; 77:1648–1655.

16. Martínez-Páramo S, Barbosa V, Pérez-Cerezales S, Robles V, Herráez MP. Cryoprotective effects of antifreeze proteins delivered into zebrafish embryos. Cryobiology. 2009; 58:128–133.

17. Chang HJ, Moon JH, Lee JR, Jee BC, Suh CS, Kim SH. Optimal condition of vitrification method for cryopreservation of human ovarian cortical tissues. J Obstet Gynaecol Res. 2011; 37:1092–1101.

18. Arav A, Rubinsky B, Fletcher G, Seren E. Cryogenic protection of oocytes with antifreeze proteins. Mol Reprod Dev. 1993; 36:488–493.

19. Lundy T, Smith P, O'Connell A, Hudson NL, McNatty KP. Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil. 1999; 115:251–262.

20. Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006; 85:Suppl 1. 1150–1156.

22. Raymond JA, DeVries AL. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A. 1977; 74:2589–2593.

23. Yang DS, Sax M, Chakrabartty A, Hew CL. Crystal structure of an antifreeze polypeptide and its mechanistic implications. Nature. 1988; 333:232–237.

24. Carpenter JF, Hansen TN. Antifreeze protein modulates cell survival during cryopreservation: mediation through influence on ice crystal growth. Proc Natl Acad Sci U S A. 1992; 89:8953–8957.

25. Chao H, Davies PL, Carpenter JF. Effects of antifreeze proteins on red blood cell survival during cryopreservation. J Exp Biol. 1996; 199(Pt 9):2071–2076.

26. Rubinsky B, Arav A, Devries AL. The cryoprotective effect of antifreeze glycopeptides from antarctic fishes. Cryobiology. 1992; 29:69–79.

27. O'Neil L, Paynter SJ, Fuller BJ, Shaw RW, DeVries AL. Vitrification of mature mouse oocytes in a 6 M Me2SO solution supplemented with antifreeze glycoproteins: the effect of temperature. Cryobiology. 1998; 37:59–66.
28. Payne SR, Oliver JE, Upreti GC. Effect of antifreeze proteins on the motility of ram spermatozoa. Cryobiology. 1994; 31:180–184.

29. Younis AI, Rooks B, Khan S, Gould KG. The effects of antifreeze peptide III (AFP) and insulin transferrin selenium (ITS) on cryopreservation of chimpanzee (Pan troglodytes) spermatozoa. J Androl. 1998; 19:207–214.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download