Abstract
Purpose
This retrospective study was done to investigate the mean platelet volume (MPV) level in patients with psoriasis vulgaris and its relationship with disease severity.
Materials and Methods
We undertook a cross-sectional study on 176 patients and 101 healthy controls to examine the association between MPV and psoriasis. Various clinical and laboratory parameters were analyzed and compared.
Results
Platelet distribution width and MPV were significantly higher in patients with psoriasis than controls. In addition, there was positive correlation between Psoriasis Area Severity Index (PASI) and MPV. When psoriasis patients were grouped into mild psoriasis (PASI<10) and moderate to severe psoriasis (PASI≥10), the MPV of the latter group was significantly elevated. Nevertheless, patients with higher MPV level (MPV≥10.4 fL) did not show higher PASI than lower MPV level (MPV<10.4 fL). MPV levels significantly decreased after improvements of psoriasis with various treatments. The variations of MPV and PASI also showed significant correlation.
Platelets are known to play important roles in inflammatory reactions and immune responses.1 Platelets can be activated by various stimuli, and its activity is known to mediate immune-inflammatory process.2 The activity of platelets is assessed by measuring various platelet-derived secretory molecules, such as adhesion proteins, growth factors, chemokines, cytokines and coagulation factors.1 Besides, mean platelet volume (MPV) and platelet distribution width (PDW) have been extensively studied and reported as platelet activation markers. Furthermore, MPV and PDW can be easily measured by automated hematology analyzer and these parameters are included in routine complete blood cell (CBC) analysis, and they are cost-effective and easy way to measure platelet activation in daily routine practice. In fact, MPV levels are shown to be related to various diseases such as cardiovascular disease, peripheral artery disease, systemic sclerosis, rheumatoid arthritis (RA), osteoarthritis, vascular dementia and Alzheimer's disease and so on.3,4,5,6
Psoriasis is a common chronic recurrent inflammatory skin disease and platelet activation has been reported to be associated with its pathogenesis.2,7 Actually, there were two previous reports on MPV level in patients with psoriasis, but their results were different and one of the studies included only small study population.8,9 Therefore, in the present study, we aimed to stuey MPV levels in psoriasis patients and healthy controls in a larger study population.
This is a retrospective study which included 176 patients with psoriasis. The participants were recruited between 1 January 2012 and 31 December 2013 at our hospital. Demographic, clinical and laboratory information were retrieved from the database of our institute. Data were also collected from subjects who have taken medical checkups at our hospital for control group.
All patients with psoriasis were fully evaluated by history taking and clinical examination. Psoriasis Area Severity Index (PASI) was measured in all patients. Blood sampling was done at initial visit, and laboratory parameters were evaluated. They included hemoglobin (Hb), whole blood cell (WBC) count, platelet count, platelet distribution width (PDW), mean platelet volume (MPV), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (Cr), uric acid, cholesterol, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Unfortunately, routine blood testing for medical checkup did not include all the parameters described, comparison between the groups in some parameters were limited. Since CRP and ESR tests were not included in medical checkup at all, both parameters could not be compared between psoriasis patients and control subjects. Platelet count, PDW, MPV, Hb, and WBC count were included in routine CBC test and they were measured using an autoanalyzer (Sysmex XE-2100, Kobe, Japan).
The MPV levels before and after psoriasis treatments were evaluated in 12 patients. Treatment modalities varied, including biologics, systemic medications, topical agents and narrow-band ultraviolet B. In addition, in order to find relationship between variations of PASI and MPV levels among 12 patients, ΔPASI [=PASI (after)-PASI (before)] and ΔMPV [=MPV (before)-MPV (after)] were calculated. In this analysis, 6 additional patients, who showed disease aggravation during follow-ups, were included.
Continuous parameters are described as means±SD and qualitative parameters as numbers and percentages. To determine differences between psoriasis patients and healthy controls, Student's t-test and Fisher's exact test were used for continuous and qualitative parameters, respectively. The correlation between PASI and other parameters were evaluated with Spearman's rank correlation coefficient. In addition, psoriasis patients were grouped according to their PASI and MPV value and they were compared. Wilcoxon signed rank test was used for analysis of MPV levels before and after psoriasis treatments. The correlation between ΔPASI and ΔMPV was also evaluated with Spearman's rank correlation coefficient.
In all our analyses, differences were considered statistically significant when p value was less than 0.05. Statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) or GraphPad Prism version 5.0 (Graphpad, San Diego, CA, USA).
The patients and healthy controls with following criteria were excluded from our study: other chronic inflammatory diseases such as RA and inflammatory bowel diseases, hypertension, diabetes, cardiovascular disorders, cancers, overt infections, hematological diseases, chronic liver or kidney diseases, autoimmune disorders.
One hundred and seventy-six psoriasis patients and 101 healthy controls were included in our study. The demographic and laboratory characteristics are shown in Table 1. There were no significant differences in age, platelet count, BUN, and uric acid between the two groups, but there were significant differences in gender, Hb, WBC count, PDW, MPV, AST, ALT, Cr, and total cholesterol between the two groups. Though gender ratio between the two groups showed significant difference, there were no statistically differences in platelet count, PDW and MPV between male and female (data not shown).
In psoriasis patients, we observed that PASI, most commonly used clinical assessment tool for disease severity, significantly correlated with MPV (r=0.189, p=0.006), ESR (r=0.165, p=0.023), CRP (r=0.152, p=0.03), and uric acid (r =0.187, p=0.007) (Fig. 1). However, PASI did not show significant correlation with age, Hb, WBC count, platelet count, PDW, AST, ALT, BUN, Cr, and cholesterol (Table 2). In addition, PASI was not different between male and female (p=0.091).
Next, we grouped patients into two groups according to their PASI score. PASI≥10 is generally regarded as moderate to severe psoriasis and PASI<10 as mild psoriasis. The number of patients with PASI<10 was 124 and 52 patients had PASI≥10. Demographic and laboratory parameters were compared between two groups (Table 3). The mean MPV level of PASI≥10 patients was statistically higher than that of PASI<10 patients (p=0.038) (Fig. 2). Unexpectedly, BUN level was increased in mild psoriasis patients compared to moderate and severe patients (p=0.045). There were no significant differences in other parameter between two groups.
Psoriasis patients were grouped according to their MPV value and PASI score was compared (Fig. 3). Subjects were grouped on the basis of MPV value 10.4 fL, since this is upper limit of reference range at our center. However, no difference of PASI was observed between two groups (p=0.2651). Other parameters were also compared between two groups, and platelet count, PDW and Hb level were found to have significant differences between two groups (Table 4). These differences do not seem to imply clinical significance, since PDW and platelet count are directly and inversely proportional to MPV, respectively. Also, hemoglobin level in each group was in normal range.
Lastly, we evaluated MPV levels of psoriatic patients before and after the treatment. They received various treatments and showed at least modest improvements of PASI after the treatments. The mean MPV values before and after the treatment were 9.97 and 9.22, respectively. Wilcoxon signed rank test were performed and there was significant decrease of MPV level in each individual after the treatment (p=0.003) (Fig. 4A). Furthermore, the variation of PASI showed significant correlation with the variation of MPV in psoriasis patients (r=0.428, p=0.038) (Fig. 4B).
Until now, there have been many studies on biomarkers in psoriasis.10 However, there are yet no clinically useful biomarkers. Several indicators of inflammation and immune response are known to be elevated in psoriasis, including CRP, E-selectin, intracellular adhesion molecule-1, haptoglobin and pro-inflammatory cytokines interleukin (IL)-1β, IL-6, IL-8, IL-12, IL-18, and tumour necrosis factor-α.11,12,13 Although there are no clinically relevant biomarkers for psoriasis, increase of these indicators in peripheral circulation definitely reflects the recent trend that psoriasis is deeply associated with various systemic diseases.14 In line with this concept, previous studies suggest that platelet activation is increased in patients with psoriasis.15,16 Among the wide array of methods measuring platelet activation, MPV and PDW are most simple parameters to estimate platelet activity.17 Especially, MPV level has been investigated in diverse diseases.17
In our current study, we also showed that MPV and PDW were significantly increased in psoriasis patients compared to healthy controls. In addition, MPV showed statistically significant correlation with PASI in psoriasis patients, but PDW did not show significant correlation. Among psoriasis patients, moderate to severe patients revealed significantly increased MPV level compared to mild patients. In addition, MPV decreased significantly after the psoriasis treatments and the variations of PASI and MPV showed significant correlation.
As described at the beginning, there are two previous reports regarding MPV in psoriasis patients, however, with conflicting results.8,9 Saleh, et al.9 recently revealed no difference of MPV between psoriasis patients and controls, whereas the study of Canpolat, et al.8 was in line with our result, which showed significantly increased MPV level in psoriasis patients compared to controls. Twenty-five and 106 psoriasis patients were analyzed in these studies, respectively. Since former study included only 25 patients, which showed no difference of MPV between psoriasis patients and controls, and studies done by Canpolat, et al.8 and our group included much more patients, we think that MPV level could be actually increased in psoriasis patients compared to controls. Moreover, in both studies, MPV value showed positive correlation with PASI. Therefore, these findings underpin significant correlation between MPV and psoriasis, which implies that activity of platelet is closely related to the pathogenesis of psoriasis.
Platelets have important role in immune responses and inflammatory reactions, not to mention their key functions in hemostasis and thrombosis.1 Various proinflammatory mediators are stored in platelets, such as adenosine diphosphate, adenosine triphosphate, serotonin, cytokines, and chemokines.18 In particular, among many chemokines and cytokines of platelets, CXCL8 and IL-1β are of interest to psoriasis researchers.19,20 CXCL8 is strongly associated with pathogenesis of psoriasis.21 Its production by peripheral blood mononuclear cells (PBMCs) was significantly increased and CXCL8 plasma levels were also elevated in psoriasis patients.22,23 IL-1β is one of the vital cytokines involved in the activation of psoriasis, and it's production by PBMCs shows positive correlation with PASI in psoriasis patients.24,25 Therefore, it will be interesting to investigate the possible contribution of CXCL8 and IL-1β from platelets to psoriasis development. In addition to proinflammatory mediators, platelets are equipped with adhesive molecules and immune receptors on their surface, which connects platelets to the immune system.1 These surface receptors include P-selectin, integreins, CD40, Toll-like receptors and chemokine receptors.1,26,27,28
With both surface receptors and stored mediators, platelets are able to respond to the variety of stimuli and release several mediators to activate other cells and inflammatory responses.
In psoriasis, alterations in platelets function have been also demonstrated by various methods other than MPV assessment. Such methods include measurements of platelet aggregation, arachidonic acid transforming capability and assessing plasma levels of P-selectin, platelet factor 4 and ß-thromoboglobulin.2,29 The mounting evidences from these studies definitely confirm that platelets are activated and involoved in pathogenesis of psoriasis. However, the precise relationship between platelet activation and pathogenesis of psoriasis has not been clearly proved. Activated platelets could participate in the development of psoriasis. On the other hand, platelets could just be bystanders and systemic inflammation of psoriasis led to their activation. Therefore, it would be interesting to find out whether activated platelets are the cause or effect of systemic inflammation in psoriasis.
In conclusion, MPV levels are increased in patients with psoriasis, especially in severe patients and the change of MPV in psoriasis individual was closely related to the clinical severity. However, precise clinical relevance of MPV and the role of activated platelets in psoriasis need to be further investigated by comprehensive propective trials and translational research, respectively.
Figures and Tables
Fig. 1
The correlations between PASI and (A) MPV, (B) ESR, (C) uric acid, (D) CRP in patients with psoriasis are depicted. PASI, Psoriasis Area Severity Index; MPV, mean platelet volume; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.

Fig. 2
Psoriasis patients are divided into mild group (PASI<10) and moderate, severe group (PASI≥10). Values and mean values of MPV are shown. PASI, Psoriasis Area Severity Index; MPV, mean platelet volume.

Fig. 3
Psoriasis patients are divided into two groups according to their MPV value (MPV<10.4 fL and MPV≥10.4 fL). PASI scores of the patients in each group are shown. PASI, Psoriasis Area Severity Index; MPV, mean platelet volume.

Fig. 4
(A) The MPV levels of psoriasis individuals were evaluated before and after the treatments. (B) The correlation between the variations of PASI (ΔPASI) and the variation of MPV (ΔMPV) was analyzed. PASI, Psoriasis Area Severity Index; MPV, mean platelet volume.

Table 1
Clinical Charateristics of Psoriasis Patients and Healthy Controls

Table 2
Correlation of PASI with Various Parameters
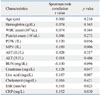
Table 3
The Comparison of Clinical Characteristics of Psoriasis Patients with PASI<10 and PASI≥10
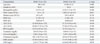
Table 4
The Comparison of Clinical Characteristics of Psoriasis Patients with MPV<10.4 fL and MPV≥10.4 fL
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI12C1672).
References
1. von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007; 100:27–40.
2. Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet function in cutaneous diseases. Platelets. 2008; 19:317–321.

3. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011; 17:47–58.

4. Pauling JD, O'Donnell VB, Mchugh NJ. The contribution of platelets to the pathogenesis of Raynaud's phenomenon and systemic sclerosis. Platelets. 2013; 24:503–515.

5. Balbaloglu O, Korkmaz M, Yolcu S, Karaaslan F, Beceren NG. Evaluation of mean platelet volume (MPV) levels in patients with synovitis associated with knee osteoarthritis. Platelets. 2014; 25:81–85.

6. Liang QC, Jin D, Li Y, Wang RT. Mean platelet volume and platelet distribution width in vascular dementia and Alzheimer's disease. Platelets. 2014; 25:433–438.

8. Canpolat F, Akpinar H, Eskioğu F. Mean platelet volume in psoriasis and psoriatic arthritis. Clin Rheumatol. 2010; 29:325–328.

9. Saleh HM, Attia EA, Onsy AM, Saad AA, Abd Ellah MM. Platelet activation: a link between psoriasis per se and subclinical atherosclerosis--a case-control study. Br J Dermatol. 2013; 169:68–75.

10. Villanova F, Di Meglio P, Nestle FO. Biomarkers in psoriasis and psoriatic arthritis. Ann Rheum Dis. 2013; 72:Suppl 2. ii104–ii110.

11. Dowlatshahi EA, van der Voort EA, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013; 169:266–282.

12. Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. The inflammatory response in mild and in severe psoriasis. Br J Dermatol. 2004; 150:917–928.

13. Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005; 2005:273–279.

15. Berrettini M, Parise P, Constantini V, Grasselli S, Nenci GG. Platelet activation in psoriasis. Thromb Haemost. 1985; 53:195–197.

16. Hayashi S, Shimizu I, Miyauchi H, Watanabe S. Increased platelet aggregation in psoriasis. Acta Derm Venereol. 1985; 65:258–262.
17. Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012; 44:805–816.

18. Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006; 99:1293–1304.

19. Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003; 10:335–350.

20. Kaplanski G, Porat R, Aiura K, Erban JK, Gelfand JA, Dinarello CA. Activated platelets induce endothelial secretion of interleukin-8 in vitro via an interleukin-1-mediated event. Blood. 1993; 81:2492–2495.

21. Pietrzak AT, Zalewska A, Chodorowska G, Krasowska D, Michalak-Stoma A, Nockowski P, et al. Cytokines and anticytokines in psoriasis. Clin Chim Acta. 2008; 394:7–21.

22. Teranishi Y, Mizutani H, Murata M, Shimizu M, Matsushima K. Increased spontaneous production of IL-8 in peripheral blood monocytes from the psoriatic patient: relation to focal infection and response to treatments. J Dermatol Sci. 1995; 10:8–15.

23. Pietrzak A, Kozioł-Montewka M, Lecewicz-Toruń B, Krasowska D. Is there any correlation between the total number of neutrophils in plasma and concentration of interleukin-8 in psoriatic patients? Med Sci Monit. 2000; 6:867–870.
24. Mee JB, Cork MJ, di Giovine FS, Duff GW, Groves RW. Interleukin-1: a key inflammatory mediator in psoriasis? Cytokine. 2006; 33:72–78.

25. Mizutani H, Ohmoto Y, Mizutani T, Murata M, Shimizu M. Role of increased production of monocytes TNF-alpha, IL-1beta and IL-6 in psoriasis: relation to focal infection, disease activity and responses to treatments. J Dermatol Sci. 1997; 14:145–153.

26. Johnston GI, Cook RG, McEver RP. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989; 56:1033–1044.

27. Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004; 104:1606–1615.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download