Abstract
Purpose
Percutaneous epidural neuroplasty (PEN) is a minimally invasive treatment. The efficacy of PEN has been relatively well investigated; however, the relationship between the clinical effectiveness of PEN and the severity of spinal canal stenosis by disc material has not yet been established. The purpose of this study was to compare clinical outcomes of PEN according to the dural sac cross-sectional area in single level disc disease.
Materials and Methods
This study included 363 patients with back pain from single level disc disease with and without radiculopathy. Patients were categorized into groups according to spinal canal compromise by disc material: Category 1, less or more than 50%; and Category 2, three subgroups with lesser than a third, between a third and two thirds, and more than two thirds. Clinical outcomes were assessed according to the Visual Analog Scale (VAS) score for back pain and leg pain and Odom's criteria at 1, 3, 6, 12, and 24 months after treatment.
Results
The demographic data showed no difference between groups according to spinal canal compromise by disc material except age (older age correlated with more spinal canal compromise). The dural sac cross-sectional area did not correlate with the VAS scores for back and leg pain after PEN in single level disc disease in Groups 1 and 2. Odom's criteria after PEN were also not different according to dural sac cross-sectional area by disc material.
Percutaneous epidural neuroplasty (PEN) is a minimally invasive therapy in which a flexible and steerable catheter is inserted directly into a region affected by a herniated disc or scar tissue that is thought to be compromising a nerve root. PEN has been used to treat intractable chronic pain that is nonresponsive to more conservative management techniques and has shown good clinical efficacy.1,2,3 Furthermore, PEN has been shown to have certain advantages over physical therapy and caudal epidural steroid injections for the treatment of chronic lower back and leg pain; namely, PEN potentially reduces the number of adhesions and the amount of fibrous tissue, which can prevent the spread of injected medications around the affected neural tissues.4,5 Thus, PEN is usually performed in patients for whom conservative treatments and conventional injections have failed.
Lumbar disc herniation (LDH) is recorded in a large number of cases of lumbar nerve root compression including those involving young patients,6,7,8 and fibrous and scar tissue around nerve roots may cause continuous neuropathic pain. Moreover, adhesions formed after spinal surgery may also result in chronic inflammation and nerve root irritation. For patients without significant neurological deficiencies, conservative treatments of lower back pain are likely sufficient for optimal short and long term outcomes. Therefore, recent studies have attempted to determine the efficacy of PEN for treating disc disease. In one such study, disc disease was classified into different types, including bulging, protrusion, extrusion, and sequestration, or by Pfirrmann disc degeneration grade according to magnetic resonance imaging (MRI) results.9 Interestingly, the efficacy of PEN according to the type of lumbar disc disease was not determined, as the authors purported that the type of lumbar disc disease is not significantly correlated with the severity of disc disease. Thus, we conducted this study to determine whether the clinical outcome of PEN is affected by the patient's dural sac cross-sectional area and also to evaluate the effectiveness and safety of PEN for treating single level LDH. To the best of our knowledge, no clinical observations of the efficacy of PEN for treating LDH have been conducted, nor have any clinical comparisons been made according to dural sac cross-sectional area with more than 12 months of follow-up.
After gaining Institutional Review Board approval (IRB No: 3190493AN01-201310-HR-006), patients meeting the following criteria were enrolled in this retrospective observational study: 1) diagnosed with single level LDH, 2) single level LDH was classified from L3/4 to L5/S1 (inclusive), 3) underwent PEN treatment at a single hospital from February 2010 to March 2011, and 4) underwent follow-up study for more than 12 months. Single level LDH diagnosis was based on MRI findings, clinical symptoms, and a neurological examination. To measure the dural sac cross-sectional area (mm2), MRI scans were analyzed and manually marginated using an imaging program (PACSPLUS PPW, Medical Standard, Seongnam, Korea). The cross-sectional area was separated into total spinal canal area and disseminated into the dural spinal canal area, as presented in Fig. 1. Patients were classified into groups according to their dural sac cross-sectional area, including 50% canal compromise (Category 1-1, less than 50%; Category 1-2, more than 50%) and 33.3% to 66.7% canal compromise (Category 2-1, less than 33.3%; Category 2-2, between 33.3% and 66.7%; Category 2-3, more than 66.7%). All patients had suffered from chronic lower back or leg pain for at least 1 month; furthermore, conservative treatments such as anti-inflammatory medications, physical therapy, and conventional epidural steroid injections had failed to provide acceptable pain relief. Additional inclusion criteria for patients were: 1) older than 18 years of age, 2) diagnosis of single level LDH with radicular pain or radiculopathy symptoms refractory to conservative treatments, 3) able to provide written consent to participate in a clinical trial, and 4) suspected presence of adhesion. Patients with multi-level LDH, lumbar stenosis, spinal cord lesions, hyaluronic acid-sensitive side effects, and uncontrolled diabetes, as well as those who had undergone previous lumbar operations, were excluded.
The Visual Analogue Scale (VAS; score range: 0 to 10, with 0 reflecting no pain) for back pain (VAS back) and leg pain (VAS leg), as well as Odom's criteria, which rate outcomes as excellent, good, fair, or poor, were used to evalute the clinical effectiveness of PEN. Its effectiveness was measured in terms of pain reduction and functional improvement before treatment (as a control), and then at 1, 3, 6, 12, and 24 months after PEN treatment. Each patient rated the average severities of his/her symptoms over the week preceding his/her visit. Successful pain relief was described as a 50% or more reduction in the patient's VAS score; good or excellent results by Odom's criteria were considered to reflect favorable outcomes.
PEN was performed with a fluoroscopy machine in a sterile operating room with monitoring equipment for blood pressure, pulse rate, and pulse oximetry. The fluoroscopy machine was adjusted over the lumbosacral area such that a caudal approach could be used for both the anteroposterior and lateral views. After appropriate positioning of the fluoroscopy machine, the needle insertion area around the sacral hiatus was determined and then injected with local anesthetic. An RK needle (Epimed International Inc., Johnstown, NY, USA) was introduced into the caudal epidural space under fluoroscopic guidance, and a lumbar epidurogram was performed using approximately 5 mL of a non-iodinated contrast agent (IOBRIX, ACCUZEN, Seoul, Korea). Filling defects were identified by examining the contrast flow pattern (Fig. 2). The placement of the needle was confirmed to be neither intravascular nor subarachnoid; if such mispositioning occurred, the needle was repositioned. After performing diagnostic epidurography, a Racz catheter was advanced through the RK needle to either the area of the filling defect or the site of pathology, as determined by MRI. Adhesiolysis was then carried out, and the needle was finally positioned in either the epidural space or into the lateral and ventral epidural space. Following satisfactory positioning of the catheter, at least 3 mL of contrast agent was injected. In the absence of subarachnoid, intravascular, or other extra-epidural filling, and when the epidural and targeted regions were satisfactorily filled, 6 mL of 0.2% preservative-free ropivacaine, containing 1500 units of hyaluronidase and 4 mL of 40% triamcinolone acetate, was injected. One hour after this procedure, 6 mL of 8% sodium chloride solution was infused over 30 minutes in the recovery room, under monitoring. The intravenous line and epidural catheter were removed, and the patient was discharged if all parameters were satisfactory. The first follow-up examination was performed 2 weeks after the procedure. During these two weeks, all participants received non-steroidal anti-inflammatory drugs and muscle relaxants to reduce procedure-related discomfort.
For statistical analysis, Student's t-test, ANOVA, and the chi-square test were conducted to determine the significance of radiological and clinical outcomes. All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA), with statistical significance defined as p<0.05.
In total, 363 patients were enrolled in this study, each with more than 12 months follow-up; moreover, 102 of these patients were followed for 24 months. Target levels were consecutive in 26 cases for L3/4, in 229 cases for L4/5, and in 108 cases for L5/S1. Among these patients, 277 cases (76.3%) were injected with single nerve root blocks prior to adhesiolysis. The demographic data of the groups are summarized in Table 1 and Fig. 3. The average age at treatment was 46.9 years old, and 48.8% of patients were male. Group 1 patients included Category 1-1 (degree of spinal canal compromise less than 50%), containing 157 patients with 12 months follow-up and 43 patients with 24 months follow-up, and Category 1-2 (degree of spinal canal compromise more than 50%), containing 206 patients with 12 months follow-up and 59 patients with 24 months follow-up. Group 2 patients included Category 2-1 (degree of spinal canal compromise less than 33.3%), containing 75 patients with 12 months follow-up and 21 patients with 24 months follow-up; Category 2-2 (degree of spinal canal compromise between 33.3% and 66.7%), containing 154 patients with 12 months follow-up and 47 patients with 24 months follow-up; and Category 2-3 (degree of spinal canal compromise more than 66.7%), containing 134 patients with 12 months follow-up and 34 patients with 24 months follow-up. In each group, old age tended to correlate with a higher degree of spinal canal compromise (mean age 42.8 years old in Category 1-1 and 48.3 years old in Category 1-2, p<0.001; mean age 43.5 years old in Category 2-1, 44.0 years old in Category 2-2, and 49.5 years old in Category 2-3, p=0.795, 0.002, and 0.001, respectively). Groups 1 and 2 exhibited similar sex ratios, manifestations of symptoms, preprocedural symptom durations, and VAS scores for back and leg pain.
Clinical assessment results are summarized in Figs. 4 and 5. The mean VAS scores of back and leg pain, respectively, were: 6.8 and 4.1 preoperatively, 1.9 and 1.2 after 1 month, 2.3 and 1.4 after 3 months, 2.2 and 1.4 after 6 months, 2.9 and 1.5 after 12 months, and 3.2 and 1.9 after 24 months (all p<0.001 compared to preoperative status, with 363 cases followed for 12 months and 102 of these followed for 24 months). Within Group 1 (Fig. 4), the mean VAS scores of back and leg pain, respectively, improved from 6.9 and 4.0 (preoperatively) to 2.8 and 1.4 (12 months after PEN treatment) and 2.8 and 1.9 (24 months after PEN treatment) in Category 1-1. Likewise, VAS scores improved from 6.8 and 4.2 (preoperatively) to 2.9 and 1.6 (12 months after PEN treatment) and 3.6 and 1.9 (24 months after PEN treatment) in Category 1-2 (all p values<0.001 between preoperative scores and scores at either 12- or 24-month follow-up examinations). Within Group 2 (Fig. 5), the mean VAS scores of back and leg pain, respectively, improved from 6.8 and 3.9 (preoperatively) to 2.8 and 1.3 (12 months after PEN treatment) and 2.6 and 2.3 (24 months after PEN treatment) in Category 2-1; from 6.8 and 4.3 (preoperatively) to 3.0 and 1.7 (12 months after PEN treatment) and 3.4 and 1.4 (24 months after PEN treatment) in Category 2-2; and from 6.8 and 4.3 (preoperatively) to 3.0 and 1.7 (12 months after PEN treatment) and 3.4 and 1.4 (24 months after PEN treatment) in Category 2-3 (all p values<0.001 between preoperative scores and scores at either 12- or 24-month follow-up examinations). No statistically significant differences in dural sac cross-sectional areas were observed between any categories or groups (Table 1).
In the present study, patients were categorized according to the extent of dural sac cross-sectional area compromise; clinical outcomes after PEN treatment did not correlate with the degree of compromise. Some studies have reported a correlation between the degree of spinal canal compromise and the extent of clinical symptoms; however, all of these studies focused exclusively on lumbar spinal stenosis.10,11,12,13 In contrast, the degree of radiographic lumbar spinal stenosis was found to have no relationship with either the extent of clinical symptoms or the Oswestry Disability Index percentage score.11,12 Regarding a possible correlation between spinal canal dimensions and the efficacy of epidural steroid injections in spinal stenosis, spinal canal dimensions have been reported to have no predictive power for assessing the success of epidural steroid injections in patients with spinal stenosis.13 Park, et al.10 also reported that PEN showed similar efficacy for the treatment of lumbar spinal stenosis; moreover, PEN did not affect the dural sac cross-sectional area. The main result obtained in the present study is consistent with all of these previous reports; namely, that different clinical outcomes are not correlated with the degree to which disc material has been compromised. These results also corroborate a previous study by our group. In this previous study, LDH type was categorized as bulging, protrusion, and extrusion, or according to Pfirrmann disc degeneration grade; however, no significant clinical differences between these types of LDH were observed during a 12-month follow-up period after PEN.9 Furthermore, surgical decompression was performed during the 12-month follow-up period for patients of the following LDH types: 7.3%, bulging; 13.8%, protrusion; 25.0%, extrusion; 30.0%, sequestration; 4.9%, Pfirrmann canal compromise grade 0; 14.0%, grade 1; 16.4%, grade 2; and 21.5%, grade 3.9,14
Percutaneous epidural adhesiolysis has been reported to be an effective method for treating degenerative central lumbar stenosis.1,15 For example, Manchikanti, et al.15 found that percutaneous epidural adhesiolysis resulted in pain relief (≥50%) in 76% of participants at 1 year following the procedure. Furthermore, a systematic review found that PEN is an effective treatment for managing chronic lower back pain (Level I to Level II-1) in patients with post-lumbar surgery syndrome.2 Pain relief achieved by PEN may result from dissolution of aberrant adhesions, as well as targeted delivery into the affected site of local anesthetics, steroids, and hypertonic sodium chloride solutions. However, our precise knowledge is currently limited regarding the management of back and lower extremity pain associated with spinal stenosis.16
There are several limitations of the present study. First, this study was a retrospective analysis. Second, this study lacked a normal control group. Hence, improvements in clinical outcomes after PEN could not be distinguished from the natural course of LDH progression. Third, the distribution of subjects according to level and type of LDH was not optimal for achieving the objectives of this study. Despite these limitations, we gleaned important clinical insights. We initially hypothesized that the efficacy of PEN would differ according to the type of LDH or Pfirrmann grade of disease; contrary to our prediction, the clinical outcomes of extrusion and sequestration LDH type were excellent. We also hypothesized that extrusion and sequestration would be worse predictors of a favorable PEN outcome. In performing PEN for patients with single level LDH, we obtained favorable results. This study indicates that PEN is a potentially suitable treatment for patients with single level LDH who do not respond to conservative treatment, thereby negating the need for lumbar surgery.
In conclusion, PEN is an effective procedure for treating single level lumbar disc herniation, without affecting the dural sac cross-sectional area.
Figures and Tables
Fig. 1
Image of a typical dural sac cross-sectional area, showing the total spinal canal area (white dashed line), canal area compromised by disc material (gray dashed line), and neural structures (dark gray dashed line).
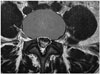
Fig. 3
Flowchart of patient stratification and follow-up after percutaneous epidural neuroplasty (PEN) due to lumbar disc herniation (LDH).

Fig. 4
Comparison of VAS scores according to the degree of spinal canal compromise (less or more than 50%) at 0, 1, 3, 6, 12, and 24 months after treatment. No statistically significant differences (p<0.05) were observed between the groups during the follow-up periods. VAS, Visual Analog Scale.

Fig. 5
Comparison of VAS scores according to the degree of spinal canal compromise (less than 33.3%, between 33.3 to 66.7%, and more than 66.7%) at 0, 1, 3, 6, 12, and 24 months after treatment. No statistically significant differences (p<0.05) were observed between the groups during the follow-up periods. VAS, Visual Analog Scale.

Table 1
Demographic Data of Patients Who Underwent Percutaneous Epidural Neuroplasty According to Their Dural Sac Cross-Sectional Areas
ACKNOWLEDGEMENTS
This study was partially supported by the Teun Teun Research Foundation and the Industrial R&D program of MOTIE/KEIT (10043086).
This research was supported by the Basic Science Research Program through the National Research foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (KRF-2011-0010067) research.
References
1. Manchikanti L, Pampati V, Fellows B, Rivera JJ, Damron KS, Beyer C, et al. Effectiveness of percutaneous adhesiolysis with hypertonic saline neurolysis in refractory spinal stenosis. Pain Physician. 2001; 4:366–373.
2. Epter RS, Helm S 2nd, Hayek SM, Benyamin RM, Smith HS, Abdi S. Systematic review of percutaneous adhesiolysis and management of chronic low back pain in post lumbar surgery syndrome. Pain Physician. 2009; 12:361–378.
3. Viesca CO, Racz GB, Day MR. Special techniques in pain management: lysis of adhesions. Anesthesiol Clin North America. 2003; 21:745–766.

4. Veihelmann A, Devens C, Trouillier H, Birkenmaier C, Gerdesmeyer L, Refior HJ. Epidural neuroplasty versus physiotherapy to relieve pain in patients with sciatica: a prospective randomized blinded clinical trial. J Orthop Sci. 2006; 11:365–369.

5. Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. A comparative effectiveness evaluation of percutaneous adhesiolysis and epidural steroid injections in managing lumbar post surgery syndrome: a randomized, equivalence controlled trial. Pain Physician. 2009; 12:E355–E368.
6. Kim DY, Oh CH, Yoon SH, Park HC, Park CO. Lumbar disc screening using back pain questionnaires: Oswestry Low Back Pain Score, Aberdeen Low Back Pain Scale, and Acute Low Back Pain Screening Questionnaire. Korean J Spine. 2012; 9:153–158.

7. Kim TW, Oh CH, Shim YS, Yoon SH, Park HC, Park CO. Psychopathological influence of lumbar disc herniation in male adolescent. Yonsei Med J. 2013; 54:813–818.

8. Lee SH, Oh CH, Yoon SH, Park HC, Park CO. Prevalence and geographic distribution of herniated intervertebral disc in Korean 19-year-old male from 2008 to 2009: a study based on Korean conscription-national and geographic prevalence of herniated intervertebral disc in Korean 19YO male-. Yonsei Med J. 2013; 54:1098–1103.

9. Ji GY, Oh CH, Lee J, Kim JH, Shin DA. 101 Clinical course after percutaneous epidural neuroplasty according to the type of single level herniated lumbar disc with 12 months follow-up. Neurosurgery. 2013; 60:153.

10. Park CH, Lee SH, Jung JY. Dural sac cross-sectional area does not correlate with efficacy of percutaneous adhesiolysis in single level lumbar spinal stenosis. Pain Physician. 2011; 14:377–382.
11. Sirvanci M, Bhatia M, Ganiyusufoglu KA, Duran C, Tezer M, Ozturk C, et al. Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J. 2008; 17:679–685.

12. Athiviraham A, Yen D, Scott C, Soboleski D. Clinical correlation of radiological spinal stenosis after standardization for vertebral body size. Clin Radiol. 2007; 62:776–780.

13. Campbell MJ, Carreon LY, Glassman SD, McGinnis MD, Elmlinger BS. Correlation of spinal canal dimensions to efficacy of epidural steroid injection in spinal stenosis. J Spinal Disord Tech. 2007; 20:168–171.

14. Pfirrmann CW, Dora C, Schmid MR, Zanetti M, Hodler J, Boos N. MR image-based grading of lumbar nerve root compromise due to disk herniation: reliability study with surgical correlation. Radiology. 2004; 230:583–588.

15. Manchikanti L, Cash KA, McManus CD, Pampati V, Singh V, Benyamin R. The preliminary results of a comparative effectiveness evaluation of adhesiolysis and caudal epidural injections in managing chronic low back pain secondary to spinal stenosis: a randomized, equivalence controlled trial. Pain Physician. 2009; 12:E341–E354.
16. Manchikanti L, Boswell MV, Singh V, Benyamin RM, Fellows B, Abdi S, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009; 12:699–802.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download