Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | Fig. 1Flow diagram of patient selection and outcomes. A total of 640 ICU patients who received CRRT for severe AKI between August 2007 and September 2009 were initially analyzed. We excluded 89 patients because they died within the first 24 hours of CRRT, were less than 18 years of age, were on chronic dialysis, or were diagnosed with terminal malignancy which was considering less than 3 month-life expectancy. In the final analysis, 551 patients were enrolled and investigated. ICU, intensive care; CRRT, continuous renal replacement therapy; AKI, acute kidney injury; non-SCT, conventional team approach; SCT, specialized CRRT team. |
 | Fig. 2Kaplan-Meier plots for cumulative 28-day mortality. 28-day all-cause mortality rates after the SCT approach were significantly reduced (log rank p=0.016). non-SCT, conventional team approach; SCT, specialized continuous renal replacement therapy team. |
Table 1
The Modules of Educational Program for SCT Nurses

| Module | Lessons | In-practice training | |
|---|---|---|---|
| I | General intensive care course | 72 hrs | 80 hrs on general ICU |
| II | CRRT specialist training course | 16 hrs | 32 hrs on general ICU |
CRRT, continuous renal replacement therapy; SCT, specialized CRRT team; ICU, intensive care unit; AKI, acute kidney injury.
Module I is corresponded to all ICU nurses. Module II is relevant to SCT nurses for CRRT. Module I includes several topics about the general management of AKI patients including nursing care for CRRT patients and practical installation and monitoring of the CRRT machine. CRRT specialist training course; basic principles and optimal prescription for CRRT, overview and practical operation of CRRT, trouble shooting in CRRT, assessment and management of failed dialysis catheter.
Table 2
The Protocol for Replacement of Electrolytes and Anticoagulation during CRRT

CRRT, continuous renal replacement therapy.
Potassium and phosphate level check 2 times per day. Phosten®; potassium phosphate. Hemosol®; hemozol B0. Definitions; 1) High risk; active bleeding, post-operative within 48 hours, low platelet count <50000/mm3, prolonged PT/PTT ≥2.0 INR/60 sec. 2) Low risk; all patients except for high risk patients. 3) High risk-1 and high risk-2 were divided according to assessment of clotting in extracorporeal system during CRRT.
Table 3
Baseline Characteristics at the Time of CRRT Start in AKI Patients Treated with CRRT
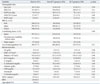
CRRT, continuous renal replacement therapy; AKI, acute kidney injury; BMI, body mass index; RIFLE, risk, injury, failure, loss, and end kidney disease; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; CCI, Charlson Comorbidity Index; MAP, mean arterial pressure; Hb, hemoglobin; WBC, white blood cell; BUN, blood urea nitrogen; Cr, creatinine; CRP, C-reactive protein; SCT, specialized continuous renal replacement therapy team.
Data are n (%), mean±SD.
Table 4
Comparisons of CRRT Outcomes between Two Groups at the 28-Day Follow-Up

Table 5
Cox Proportional Hazards Analysis for 28-Day Mortality Based on SCT

| Compared with non-SCT management | |||
|---|---|---|---|
| HR | 95% CI | p value | |
| Model 1 | 0.77 | 0.63-0.95 | 0.016 |
| Model 2 | 0.76 | 0.58-0.98 | 0.039 |
| Model 3 | 0.87 | 0.66-0.92 | 0.041 |
| Model 4 | 0.91 | 0.69-0.94 | 0.046 |
SCT, specialized CRRT team; CRRT, continuous renal replacement therapy; non-SCT, conventional team approach; HR, hazard ratio; CI, confidence interval; MAP, mean arterial pressure; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; RIFLE, risk, injury, failure, loss, and end kidney disease.
Model 1: unadjusted relative risk. Model 2: adjusted for age, gender, MAP, APACHE II score and SOFA score, CRP. Model 3: adjusted for Model 2 plus, hemoglobin, albumin, eGFR, total cholesterol, total bilirubin. Model 4: adjusted for Model 3 plus RIFLE and contributing factors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download