Abstract
Purpose
The Maze procedure has shown excellent efficacy in the elimination of atrial fibrillation (AF); however, little is known about the quality of functional recovery in the left atrium (LA) following successful sinus rhythm conversion by the Maze procedure.
Materials and Methods
We prospectively enrolled 12 patients (aged 52.5±10.1 years, 1 female) with valvular AF undergoing mitral valve surgery combined with the Maze procedure. Parameters of LA function in three anatomic compartments [anterior, posterior, and LA appendage (LAA)] were evaluated using electrocardiography-gated dual-source cardiac CT at one month and at six months after surgery. Twelve subjects matched by age, gender, and body surface area served as controls.
Results
At one month after surgery, ejection fraction (EF) and emptying volume (EV) of the LA were 14.9±7.4% and 21.3±9.7 mL, respectively, and they were significantly lower than those of the control group (EF, 47.9±11.2%; EV, 46.0±10.7%; p<0001). These values did not significantly change throughout late periods (p=0.22 and 0.21, respectively). Functional contributions of the anterior, posterior, and appendage compartments (EV of each compartment/overall EV) were 80.4%, -0.9%, and 20.5%, respectively, for those with LAA preservation (n=6); 100.1%, -0.1%, and 0% for those with LAA resection (n=6; p<0.05); and 62.2%, 28.2%, and 9.7% in the control subjects (p<0.001).
The Maze procedure has been recognized as the most effective method of restoring normal sinus rhythm in patients with chronic atrial fibrillation (AF), and has been found to result in symptom improvement, improved hemodynamics, a reduction in thromboembolic events, and even improved survival.1,2,3,4,5,6,7 However, little is known about how much of atrial contractile functional recovery can be achieved by successful sinus rhythm conversion with the Maze procedure. This is partly attributable to difficulties in evaluating atrial functions with conventional imaging modalities, such as echocardiography, in which the complex three-dimensional morphology of the atria hinders comprehensive evaluation of contractile functions. Furthermore, deciding between whether to preserve or amputate the left atrial appendage (LAA) during surgical ablation of AF remains controversial, and due to a lack of reliable scientific evidence, management thereof is left to the discretion of the surgeon. Recently, multi-slice cardiac computed tomography (CT) has been reported as a promising tool for the assessment of left atrium (LA) volume and function in various clinical settings,8,9,10,11 making comprehensive evaluation of the atria possible in the setting of surgical ablation of AF.
In this study, we aimed to evaluate postoperative left atrial function using dual-source cardiac CT in patients with valvular atrial fibrillation undergoing the Maze procedure. For secondary analysis, we sought to evaluate the impact of LAA resection versus preservation during the surgery on postoperative LA function.
This prospective observational study included patients with valvular AF who were scheduled for mitral valve (MV) surgery combined with the Maze procedure. Exclusion criteria were as follows: 1) contraindicated to receive radiocontrast media; 2) severe LV dysfunction [LV ejection fraction (EF) <40%]; 3) valvular dysfunction≥a moderate degree, other than functional tricuspid insufficiency; 4) presence of LA thrombus; 5) requirement of multi-vessel coronary bypass, aortic replacement, or correction for congenital heart defects; and 6) history of previous cardiac surgery.
At one and six months after surgery, the participating patients were scheduled for functional evaluation of LA using dual-source cardiac CT. These parameters included LA volume (both systolic and diastolic), EF, and emptying volume (EV). For secondary analysis, we randomly assigned patients into two groups according to resection or preservation of the LAA. For a control group, 12 subjects matched for age, gender, and body surface area were selected out of normal subjects who underwent cardiac CT as part of a general health checkup at the Asan Medical Center during the same study period.
This study was approved by our Institutional Review Board, and informed consent was obtained from all patients.
The AF ablation was performed using an argon-based flexible CryoAblation system, SurgiFrost (Medtronic, Minneapolis, MN, USA), in all patients. After snaring down the superior and inferior vena cavae, right atrial ablation was preformed through an oblique right atriotomy on a beating heart. Endocardial ablation comprised a cavo-tricuspid isthmus isolation lesion and a line from the isthmus lesion to the superior vena cava.
After aortic cross-clamping, MV exposure was obtained through a LA incision. The LA ablation was done endocardially before the MV procedure, which included a single box lesion for pulmonary veins isolation, a line from the pulmonary isolation lesion to the LAA, and another line from the pulmonary isolation lesion to the MV annulus, posteriorly. Additional epicardial coronary sinus ablation was done on the opposite side of the MV annular lesion. LA size was reduced by resection of redundant atrial tissue between the inferior pulmonary veins and the posterior mitral annulus.12 In cases of concomitant tricuspid valve repair, 26-30-mm commercially available rings (Edwards MC3 Ring, Edwards Lifesciences, Irvine, CA, USA) were used for annuloplasty.
At one and six months after surgery, assessment of LA functional parameters was performed using ECG-gated second generation dual source cardiac CT (Somatom Definition Flash, Siemens, Erlangen, Germany) for those with restored sinus rhythm (expected number of patients at each point=10). Tube voltage and tube current-time product were adjusted by body size, and scan parameters were as follows: tube voltage, 80-120 kV; tube current-time product, 185-380 mAs; collimation, 128×0.6 mm; spatial resolution, 0.3 mm; gantry rotation time, 280 sec; and a temporal resolution of 75 ms. A bolus of 70-90 mL of contrast agent was administered at a rate of 4.0 mL/s, followed by 40 mL of saline chaser. The scan delay was determined by the bolus-tracking method (region of interest, ascending aorta; attenuation threshold level, 100 HU; scan delay, 8 seconds). Retrospective ECG-gated spiral scan was done, and ECG-based tube current modulation was applied to reduce radiation doses. Four-dimensional multiphase cardiac CT data were transferred to an external workstation (Advantage Workstation Server, GE, Milwaukee, MI, USA) for post-processing.
We obtained three-dimensional volumetric data for the entire LA (Fig. 1A). The total LA volume was defined as the volume from the MV to the posterior wall of the LA, and it was divided into the anterior, posterior, and LAA compartments (Fig. 1). To divide each component of LA, we generated volume rendering images of LA using a dedicated semi-automatic tool (Auto Ejection Fraction, GE, Milwaukee, MI, USA) in the workstation. This software provides a threshold-based LA segmentation function. By rotating the volume rendering images of LA, anatomic landmarks of each LA component were evaluated, and LAA and posterior compartments of LA were clipped manually on the volume rendering image.
The LAA was defined as the volume from the tip of the LAA to the ridge between the left superior pulmonary vein and the LAA.8,13 The LA proper was defined by subtracting the LAA volume from the total LA volume (Fig. 1B). The boundary that divides the LA proper into the anterior and posterior compartments was defined as follows: the anterior border of the ostia of the four pulmonary veins was identified, and the curvilinear line that connected them was delineated. The anterior compartment was defined as the volume from the anterior wall of the LA to the boundary of the ostia of the four pulmonary veins (Fig. 1C). The posterior compartment was defined by subtracting the anterior compartment from the LA proper. The following parameters were evaluated in each compartment of the LA, pre- and post-operatively: 1) maximum LA volume, 2) minimum LA volume, 3) EV, and 4) EF. The maximum LA volume was defined as the volume at the end of atrial diastole and immediately before MV opening. The minimum LA volume was defined as the volume after atrial emptying at the end of atrial systole. The EV was calculated as the difference between the maximum and minimum LA volume. The EF was calculated as the EV divided by the maximum LA volume.14
The rhythm follow-up protocol in this study was as follows: 1) continuous monitoring during intensive care unit stay, 2) daily ECG and electroatriography during hospital stay, 3) a 24-hr Holter monitoring before hospital discharge if the patient maintained "free-of-AF status," and 4) snap EKGs at 1 month, 3 months, and 6 months post-surgery.
A power calculation estimated that approximately 10 patients were required to achieve a minimum power of 90% to detect a 20% difference in LA EF between the study and normal subjects with a 90% confidence interval, given that normal LA EF is 42.9±6.6% (42.89%).8 Assuming a study dropout rate of 15%, including those who did not restore sinus rhythm or were contraindicated to undergo postoperative CT evaluation (i.e., postoperative renal failure), around 12 study subjects were required.
Categorical variables are presented as frequencies and percentages, and were compared using Fisher's exact test. Continuous variables are expressed as mean±SD or medians with ranges, and were compared using the Wilcoxon signed rank test or the Mann-Whitney U test, as appropriate. Changes in maximal and minimal LA volumes were investigated using repeated-measures ANOVAs.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
From March through May 2012, a total of 12 patients (aged 52.5±10.1 years, 1 female) with valvular AF were enrolled. We randomly assigned these patients to resection (n=6) or preservation (n=6) of the LAA. Baseline characteristics of the patients are summarized in Table 1. There was no operative mortality or morbidity.
Overall postoperative rhythm statuses during the study period are detailed in Table 2. Overall, 10 patients (83.3%) were free of AF and off anti-arrhythmic medications after 6 months of surgery. The incidences of postoperative bradyarrhythmia (1 vs. 1) and AF (2 vs. 2), as well as requirement of anti-arrhythmic medications (1 vs. 2), were not different between the LAA resection and preservation groups. The baseline demographic variables of the control group were well matched with those of the study group, including age (53.2±11.4 years vs. 52.5±10.1 years, p=0.88), BSA (1.90±0.14 m2 vs. 1.83±0.14 m2, p=0.30), and gender distribution (females: 1/12 vs. 1/12, p=1.0).
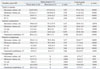
Except for two patients who showed AF, 10 patients (with sinus rhythm) underwent cardiac CT evaluation at one month after surgery. EF of the entire LA chamber was 16.8±6.3%, and this value was significantly lower than that in the control subjects (47.9±11.2%, p<0.001). An EV of 21.3±9.7 mL was also significantly lower than that in the control subjects (46.0 control subjects 10.7 mL, p<0.001). At six months after surgery, CT data from 11 patients who showed restored sinus rhythm were obtained. CT data at 6 months were available in 11 patients who showed restored sinus rhythm, and all LA functional parameters showed similar results as those at one month (Figs. 2 and 3). Details of LA functional parameters at six months for each compartment are shown in Table 3. EV, EF, and functional contribution (EV of a compartment/EV of total LA chamber) for the anterior compartment were significantly higher than those for the posterior compartment (p<0.001 for all values), in which the contributions of the posterior compartment to overall LA contraction were trivial (-0.9 to -0.1% of overall function) (Fig. 4). Although LAA contributed a significant proportion to overall LA contractile function (20.5±14.2%) in patients with LAA preservation, overall EV and EF of the LA were not significantly different between LAA preservation and resection groups (p=0.58 and 0.86, respectively).
During the postoperative period, echocardiographic evaluation revealed LV EF of 53.5±4.6% (range, 53-64%; p=0.86), LV systolic dimension of 35.6±4.5 mm (range, 30-44 mm; p=0.007), and LV diastolic dimension of 53.8±4.4 mm (range, 45-61 mm; p=0.38), showing comparable LV systolic function with that in preoperative settings.
In this prospective observational study, we found that the Maze procedure was effective in restoring sinus rhythm in valvular AF patients; however, even after successful recovery of sinus rhythm, functional recovery of the LA was significantly lower than that in normal subjects. Additionally, we found that LAA contributed to a significant proportion of LA contractile function after the Maze procedure, although a valid comparison between preservation and resection procedures could not be made because of the small number of patients in this study.
Although surgical ablation of AF is primarily targeted toward the elimination of AF, its benefits secondarily extend to improving hemodynamic performance by the incorporation of synchronous atrial kicking into the cardiac output.1,15,16 To date, however, the extent of functional recovery of the atria by a successful Maze procedure is poorly understood. This is mainly attributed to a limited ability to precisely evaluate LA geometry and booster function with the use of conventional two-dimensional echocardiography due to the complex three-dimensional shape of the LA.17,18,19 Furthermore, since diastolic inflow measurements, such as E/A ratio, are heavily affected by several factors,17,18 assessment by echocardiography holds intrinsic limitations in evaluating LA contractile function. In these regards, multi-slice cardiac CT has been reported to be a precise tool for the assessment of LA volume and booster functions in various clinical settings.8,9,10,11
The LA is a complex three-dimensional structure that comprises three anatomic compartments of three different embryologic origins: the anterior LA, the posterior or venous LA, and the LAA.13,20 Since the structure of the LA differs in each compartment, and, consequently, the functional contribution of each compartment may also differ, it is important to understand the functional nature of each LA compartment during AF ablation. Although several research groups reported on the roles of the LAA as a contractile component and as a main source of thrombus formation in AF patients,21,22 functional assessments of the other compartments have not been reported for surgical ablation of AF.
In the present study, LA booster function, represented by EF and EV, was much lower than that of normal subjects reported in the literature.8 This finding may be attributable to several reasons: first, since cryoablation does not produce sharp injuries, and typically tends to freeze surrounding tissue together, transient or permanent collateral cryo-damages may have occurred in adjacent atrial tissue, resulting in functional damage to the atrium. Furthermore, as the functional contribution of the posterior compartment was nearly zero in this study, isolation of the posterior compartment by creating a box lesion may have adversely affected LA function. For instance, an EF of the posterior compartment of -0.9 to -0.1% is quite distinctive, compared with normal populations, in which it is reported to be around 20-40% (28.2% for control subjects in this study).8 In these regards, creating separate left and right pulmonary-vein isolation lesions connected by another linear lesion, rather than a large posterior box lesion, may be a more reasonable option through which to better preserve posterior compartment function of the LA.
Finally, but most importantly, the differences in LA function between the subject patients and normal subjects most likely stem from differences in baseline pathologic features of the atrial tissue. Longstanding volume and/or pressure overload to the LA secondary to MV diseases results in substrate changes in the atrium, the pathologic process of which involves atrophy of atrial myocytes, excessive interstitial fibrosis, and overall thinning and dilatation of the atrial wall.23,24 These pathologic changes very likely limit the extent of atrial booster function, even with optimal preservation of the atrial contractile component during the Maze procedure. This hypothesis is supported by a previous study that quantitatively assessed LA booster function after the Maze procedure.9 In the cited study, the authors examined 14 consecutive patients undergoing concomitant MV surgery and the Maze procedure, and found out that LA EF was only 15% following the Maze procedure, which was significantly smaller than that of patients with sinus rhythm who underwent coronary bypass (p=0.004). The EF of 15% corresponds well to the result of the present study.
Management of LAA during surgical ablation of AF is another important issue to address. Since ectopic foci of AF may arise from the LAA,25 its resection may improve the efficacy of AF elimination. Furthermore, given that a significant proportion of patients receiving the Maze procedure fail to achieve AF elimination and that the LAA is the most common site of thrombus formation and embolization, amputation of the LAA may reduce the risk of thromboembolic complications or the requirement for long-term anticoagulation therapy.27 Preservation of the LAA, on the other hand, may benefit patients by conserving LA transport function, since the LAA has been reported to play significant roles in the contribution of overall atrial contractile function.8
The secondary objective of the present study, therefore, was to compare resection versus preservation of the LAA in regards to postoperative LA function. Since the number of patients for this study had been determined based on the assessment of overall LA function in comparison with a normal reference value, the sample size was too small to draw a statistically meaningful comparison between the two groups (resection vs. preservation of LAA). Nevertheless, we found out that the contribution of the LAA to overall LA booster function was 21.5±16.1% for patients who underwent LAA preservation, which was significantly higher than that of the posterior compartment (p<0.001). We believe that these findings open the possibility for superior functional restoration of the LA by preservation of the LAA, and this hypothesis needs to be tested by further studies. LA functional values presented in this study may help calculate sample sizes required to compare LA functions in prospective randomized studies.
AF related cardiomyopathy is another issue related to the treatment of AF. AF may lead to tachycardia-induced cardiomyopathy with resultant LV systolic dysfunction.26,27 Tachycardia-induced cardiomyopathy is commonly thought to be associated with ventricular rates of 100 to 120/min in chronic phases. Even in patients with AF and well-controlled resting heart rates, minimal activity may frequently induce rapid ventricular beating, which can be associated with the development of tachycardia-induced cardiomyopathy.28 Meanwhile, elimination of AF has been suggested to restore LV function by blocking this pathological process. For instance, a group reported that surgical AF ablation in patients with lone AF improved LV ejection and reduced LV end-diastolic diameter, which appeared immediately after surgery and was maintained through late periods.29 Recently, Nedios showed via the evaluation of 69 patients with impaired LV function undergoing catheter AF ablation that both the elimination of AF and baseline heart rate following the procedure were independent predictors of a marked improvement in LV function.
Although the number of patients enrolled in this study was calculated based on pertinent statistical assumptions, it was relatively small, limiting the drawing of robust conclusions. Also, since the subject population in this study was confined to patients with valvular AF, the study results may not be generalizable to patient with AF of other etiologies. The heterogeneity of surgical procedures in this study (e.g., sternotomy vs. mini-thoracotomy and valve replacement vs. repair) may be another limitation of this study. Also, the control subjects were fundamentally different from those receiving MV surgery in the presence of AF, and therefore, the comparisons of outcomes between these two groups may not be fair. Nevertheless, the study design was set as such because the primary aim of this study was to describe the nature of functional recovery in the LA after the Maze procedure in comparison to normal LA function. Finally, while the sample size was too small to evaluate the effects of resection versus preservation of the LAA in this study, our results could be of use as references for further studies to determine sample sizes required to fairly compare the effects of LAA resection in the setting of Maze surgery.
In conclusion, LA function was significantly decreased after successful sinus rhythm conversion by the Maze procedure in patients with valvular AF undergoing MV surgery, which persisted throughout late postoperative period. Conclusions regarding preservation versus resection of the LAA in terms of clinical, cardiac rhythm-, and atrial functional outcomes await further studies involving larger populations.
Figures and Tables
Fig. 1
Three-dimensional measurement of LA volumes using volume-rendering imaging. Green demarcation lines indicate the extents of measurement for each compartment including (A), entire LA (right-anterior view); (B) LAA-extracted LA (right-anterior view); and (C) anterior compartment of the LA (right lateral view). RUPV, right upper pulmonary vein; LA, left atrium; LAA, left atrial appendage; RA, right atrium; LV, left ventricle; PA, pulmonary artery.
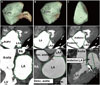
Fig. 2
Changes in overall LA volume at each time point. (A) Maximal volumes. (B) Minimal volume. LA, left atrium; LAA, left atrial appendage.

Fig. 3
LA contractile function at one and six months after surgery. (A) LA emptying volume. (B) LA ejection fraction. LA, left atrium; LAA, left atrial appendage.

Fig. 4
Booster functional contribution of each left atrial chamber (anterior, posterior, and LAA) at one and six months after surgery. LAA, left atrial appendage.

Table 1
Baseline Characteristics of Patients
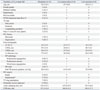
NYHA, New York Heart Association; AF, atrial fibrillation; MV, mitral valve; LV, left ventricle; EF, ejection fraction; LVIDs, left ventricular systolic dimension; LVIDd, left ventricular diastolic dimension; LA, left atrium; TR, tricuspid regurgitation; CPB, cardiopulmonary bypass; LAA, LA appendage.
*All were mechanical prosthetic implantation.
Table 2
Postoperative Cardiac Rhythm Status

Table 3
Postoperative Left Atrial Functional Parameters at 6 Months (11 Patients with Sinus Rhythm)
References
1. Ad N, Henry L, Hunt S. The impact of surgical ablation in patients with low ejection fraction, heart failure, and atrial fibrillation. Eur J Cardiothorac Surg. 2011; 40:70–76.

2. Bando K, Kasegawa H, Okada Y, Kobayashi J, Kada A, Shimokawa T, et al. Impact of preoperative and postoperative atrial fibrillation on outcome after mitral valvuloplasty for nonischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2005; 129:1032–1040.

3. Cohn LH, Couper GS, Aranki SF, Rizzo RJ, Kinchla NM, Collins JJ Jr. Long-term results of mitral valve reconstruction for regurgitation of the myxomatous mitral valve. J Thorac Cardiovasc Surg. 1994; 107:143–150.

4. Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg. 1999; 118:833–840.

5. Cox JL, Boineau JP, Schuessler RB, Ferguson TB Jr, Cain ME, Lindsay BD, et al. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991; 266:1976–1980.

6. Gillinov AM, Sirak J, Blackstone EH, McCarthy PM, Rajeswaran J, Pettersson G, et al. The Cox maze procedure in mitral valve disease: predictors of recurrent atrial fibrillation. J Thorac Cardiovasc Surg. 2005; 130:1653–1660.

7. Kim JB, Moon JS, Yun SC, Kim WK, Jung SH, Choo SJ, et al. Long-term outcomes of mechanical valve replacement in patients with atrial fibrillation: impact of the maze procedure. Circulation. 2012; 125:2071–2080.

8. Park MJ, Jung JI, Oh YS, Youn HJ. Assessment of the structural remodeling of the left atrium by 64-multislice cardiac CT: comparative studies in controls and patients with atrial fibrillation. Int J Cardiol. 2012; 159:181–186.

9. Yamanaka K, Fujita M, Doi K, Tsuneyoshi H, Yamazato A, Ueno K, et al. Multislice computed tomography accurately quantifies left atrial size and function after the MAZE procedure. Circulation. 2006; 114:1 Suppl. I5–I9.

10. den Uijl DW, Tops LF, Delgado V, Schuijf JD, Kroft LJ, de Roos A, et al. Effect of pulmonary vein anatomy and left atrial dimensions on outcome of circumferential radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol. 2011; 107:243–249.

11. Kataoka A, Funabashi N, Takahashi A, Yajima R, Takahashi M, Uehara M, et al. Quantitative evaluation of left atrial volumes and ejection fraction by 320-slice computed-tomography in comparison with three- and two-dimensional echocardiography: a single-center retrospective-study in 22 subjects. Int J Cardiol. 2011; 153:47–54.

12. Kim JB, Bang JH, Jung SH, Choo SJ, Chung CH, Lee JW. Left atrial ablation versus biatrial ablation in the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2011; 92:1397–1404.

14. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006; 47:2357–2363.
15. Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. 1991; 84:40–48.

17. Feinberg MS, Waggoner AD, Kater KM, Cox JL, Lindsay BD, Pérez JE. Restoration of atrial function after the maze procedure for patients with atrial fibrillation. Assessment by Doppler echocardiography. Circulation. 1994; 90(5 Pt 2):II285–II292.
18. Yashima N, Nasu M, Kawazoe K, Hiramori K. Serial evaluation of atrial function by Doppler echocardiography after the maze procedure for chronic atrial fibrillation. Eur Heart J. 1997; 18:496–502.

19. Jessurun ER, van Hemel NM, Kelder JC, Defauw JA, Brutel de la Rivière A, Ernst JM, et al. The effect of maze operations on atrial volume. Ann Thorac Surg. 2003; 75:51–56.

20. Anderson RH, Cook AC. The structure and components of the atrial chambers. Europace. 2007; 9:Suppl 6. vi3–vi9.

21. Cox JL. The central controversy surrounding the interventional-surgical treatment of atrial fibrillation. J Thorac Cardiovasc Surg. 2005; 129:1–4.

22. Lönnerholm S, Blomström P, Nilsson L, Oxelbark S, Jideus L, Blomström-Lundqvist C. Effects of the maze operation on health-related quality of life in patients with atrial fibrillation. Circulation. 2000; 101:2607–2611.

23. Henry WL, Morganroth J, Pearlman AS, Clark CE, Redwood DR, Itscoitz SB, et al. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation. 1976; 53:273–279.

24. Bailey GW, Braniff BA, Hancock EW, Cohn KE. Relation of left atrial pathology to atrial fibrillation in mitral valvular disease. Ann Intern Med. 1968; 69:13–20.

25. Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003; 107:3176–3183.

26. Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997; 29:709–715.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download