Abstract
Purpose
This study was to evaluate the characteristics of selective spinal anesthesia using 1 mg of bupivacaine combined with fentanyl or sufentanil in elderly patients undergoing transurethral resection of prostate.
Materials and Methods
Fifty-six patients were randomized into two groups. The Fentanyl group received 0.5% hyperbaric bupivacaine 0.2 mL+fentanyl 20 µg+5% dextrose 1.4 mL, and the Sufentanil group received 0.5% hyperbaric bupivacaine 0.2 mL+sufentanil 5 µg+5% dextrose 1.7 mL intrathecally. Intraoperative and postoperative characteristics were evaluated. Patient satisfaction was assessed postoperatively.
Results
Twenty-six patients in each group completed the study. The median peak sensory block level was similar between two groups, but sensory regression time was longer in the Sufentanil group than the Fentanyl group (p=0.017). All patients were able to move themselves to the bed without any aid when they arrived at the admission room. Pain scores were lower in the Sufentanil group than the Fentanyl group at postoperative 6, 12, and 18 hours (p=0.001). Compared to the Fentanyl group, the Sufentanil group required less postoperative analgesia (p=0.023) and the time to the first analgesic request was longer (p=0.025). Twenty-four of 26 patients (92.3%) in each group showed "good" satisfaction level.
Conclusion
Selective spinal anesthesia using 1 mg of bupivacaine with fentanyl or sufentanil provided appropriate sensory block level with spared motor function for transurethral resection of the prostate in elderly patients. Intrathecal sufentanil was superior to fentanyl in postoperative analgesic quality.
Benign prostatic hyperplasia (BPH) has a high prevalence of over 60% in males older than 60 years;1 whereas the prevalence thereof up to 90% in patients older than 80 years.2 Since BPH is associated with old age, surgery is usually performed in patients with various comorbidities. The comorbidity rate is over 60% in elderly patients who undergo transurethral resection of the prostate (TURP), thus directly affecting perioperative morbidity and mortality after TURP.3,4,5,6
Spinal block, the most common anesthetic technique for TURP, has been conventionally shown to block up to 10th thoracic dermatome.7 Pain sensations from the prostate and bladder are conducted by sympathetic (S2-4, T11-L2) and parasympathetic outputs (S2-4). The autonomic nerve supply to the penile urethra also comes from the S2-S4, and the other part of perineum is supplied by somatic branches of the pudendal nerve (S2-4).8,9 Considering the pain pathway and differential spinal blockade pattern, sensory block up to T12 is adequate for TURP, as Beers, et al.10 suggested. Moreover, sensory block levels are approximately 3-4 dermatomes higher in those of older age than in young adults.11,12 The sympathetic block level is generally 1-4 segments higher than the analgesia level.13,14 Thus, in elderly patients, high sympathetic block is frequent during spinal block, which may explain frequent cardiovascular side effects, compared to young adults.12,14 Therefore, it is important to restrict the block level in elderly patients.
Selective spinal anesthesia (SSA) is defined as "the practice of employing minimal doses of intrathecal agents so that only the nerve roots supplying a specific area and only the modality that require to be anesthetized are affected."15 Thus, SSA is more appropriate in elderly patients.7 Additionally, rapid recovery with spared motor function is a tremendous boost to patient satisfaction.16 To the best of our knowledge, 1.5 mg of bupivacaine is the lowest dose for spinal anesthesia ever reported,17 but the effectiveness of sole bupivacaine is controversial.18 Notwithstanding, it is well known that co-administered intrathecal opioid enhances anesthetic quality, duration, and success rate when low-dose local anesthetic is used.4,19,20 Intrathecally administered fentanyl or sufentanil can rapidly be diffused into the spinal cord and bound to opioid µ receptor due to their lipophilic essentials.21 When they are given with even low dose bupivacaine, the anesthetic quality of spinal block is enhanced as well.4,19,20,21
Thus, we designed this prospective, randomized, double-blind study to evaluate the sensory block level and anesthetic characteristics after spinal block using 1 mg of bupivacaine with fentanyl or sufentanil in elderly patients who were undergoing TURP. In addition, we compared the quality of the block with co-administered fentanyl or sufentanil.
This study was approved by the Institutional Research Board of Yonsei University Health System (IRB number: 4-2011-0162) and registered at www.ClinicalTrials.gov (ref: NCT 01608334). Fifty six patients who underwent elective TURP at the Severance Hospital were prospectively enrolled between August 2011 and January 2012. Written informed consent was obtained from all patients. Patients with histories of back surgery, infection in the back, coagulopathy, hypersensitivity to local anesthetics or opioids, mental disturbance, or neurological disease were excluded.
Prior to this study, we performed a dose-finding study using an up-and-down method to investigate minimum effective dose of bupivacaine necessary to obtain sensory block of T12 level for TURP. As 4 mg of bupivacaine was shown to be a successful dosage in our previous study,4 we started from 3 mg of bupivacaine. The drug solution (total 2 mL) consisted of 0.5% hyperbaric bupivacaine (Heavy; Hana Pharm., Seoul, Korea), fentanyl 20 µg or sufentanil 5 µg, and 5% dextrose solution. When 3 mg was successful, the dosage was lowered to 2 mg on the next patient; if it failed, the dosage was increased to 4 mg on the next patient (Fig. 1). During the study, spinal block with 1 mg of bupivacaine was consecutively successful in 4 patients. We decided not to further decrease the dosage due to ethical reasons and performed an interim analysis. Upon statistical analysis of the data, the ED50 and ED95 of bupivacaine were 0.97 mg (CI 83%) and 1.05 mg (CI 95%), respectively, in the sufentanil group (Group S), while a fitting error occurred in the fentanyl group (Group F) and none of the calculations showed up. Hence, we redesigned the study to evaluate the sensory block level and anesthetic characteristics after spinal block using 1 mg of bupivacaine combined with fentanyl or sufentanil.
Patients were allocated to two groups by using a computer-generated sequence of numbers and sealed envelopes. An independent investigator prepared the drugs (total of 2 mL), 0.5% hyperbaric bupivacaine and opioids, by using 1 mL insulin syringes to minimize an aberration of the amount of drug. These solutions were mixed with a 5% dextrose solution in 2 mL sterile syringes and given to the anesthesia administrator. All drugs were prepared sterilely at room temperature. The anesthesia administrator, patients, and the outcome investigator were blinded to the allocated groups. Group F received 0.5% hyperbaric bupivacaine 1 mg (0.2 mL)+fentanyl 20 µg (0.4 mL)+5% dextrose solution 1.4 mL (total 2 mL) and Group S received 0.5% hyperbaric bupivacaine 1 mg (0.2 mL)+sufentanil 5 µg (0.1 mL)+5% dextrose solution 1.7 mL (total 2 mL) intrathecally. Drug densities were measured in five identical samples of drug solution used in each group using Gay-Lussac-Hofmann's method at room temperature. The mean densities of fentanyl-mixed solution and sufentanil-mixed solution were 1.0172±0.0003 g mL-1 and 1.0182±0.0006 g mL-1, respectively.
No premedication was given. We administered 300 mL of lactated Ringer's solution to each patient before performing the spinal anesthesia as a routine process. After standard monitoring of blood pressure, electrocardiogram, and SpO2, spinal puncture was performed through the midline approach at L3-4 or L4-5 with a 25 G Quincke needle in a lateral decubitus position. After checking the free flow of clear cerebrospinal fluid, the drug was administered over 10 seconds and barbotage was performed gently. Patient was immediately placed in a supine horizontal position and then placed in a 10° head-down position. Before anesthesia, patients were informed about the methods of cold, sensory, and motor assessments by the anesthesia administrator. The cold and sensory block levels were assessed bilaterally on the mid clavicular line using an ice cube and pinprick (22 G hypodermic needle) every 2 minutes until the peak level was reached. The peak block level was defined as the level that persisted over four consecutive tests. When the peak sensory and cold blocks were obtained, the loss of touch sense to light finger touch was assessed. And the motor block was measured using a modified Bromage scale (1=complete motor block; 2=able to move feet only; 3=able to move feet and bend knees; 4=able to perform a straight leg raise <30°; 5=able to perform a straight leg raise >30°).22 Three expert urologists performed all the surgeries separately. Plain lubricant without local anesthetic was used to insert the endoscope. Mean arterial pressure (MAP), heart rate (HR), and SpO2 were measured every 5 minutes until the end of surgery. We planned to treat hypotension (decreased to MAP <30% of baseline) or bradycardia (<45 bpm) with ephedrine or atropine. Considering systemic absorption of irrigating fluid, small amounts of intravenous fluid were maintained during operation.
In the post anesthetic care unit (PACU), a blinded investigator assessed motor block upon regression of two sensory dermatomes. Prior to discharge from the PACU, the patients were asked of their satisfaction level: good (I would choose this technique if I needed a re-operation), fair (was satisfactory, but I would reconsider this method if I needed a re-operation), or poor (not satisfactory). When the patients arrived at the ward, they were allowed to move to the bed by themselves without aid if they did not perceive any changes in motor power.23 An outcome investigator assessed the pain scores using a verbal numerical rating scale for pain (0=no pain and 10=worst pain imaginable) every 6 hours for 36 hours post-operatively. Analgesic requirements, first analgesic request time, and side effects, such as nausea/vomiting, post-dural puncture headache (PDPH), or pruritus, were evaluated.
A sample size calculation was performed based on our previous study,5 in which mean±SD difference of dermatomes in the peak analgesia level was 2±2.5. To detect differences in two levels between the two groups (two-sided α of 0.05 and power of 80%), 25 patients were required in each group. We included 28 patients per group with a possible 10% dropout rate.
Statistical analysis was performed using IBM SPSS Statistics 19.0 (SPSS Inc., Chicago, IL, USA) and R version 3.0.1. Patient characteristics, time to peak block level, and time to two-segment regression were analyzed using Student's t-test. Inter-group differences in peak block levels, maximum motor block, time to the first analgesic requirement, and post-operative pain scores were compared using the Mann-Whitney U test. Categorical data (analgesic request and side-effects) were compared using the χ2 test. Statistical significance was considered when p<0.05 and was not mentioned when p value was not significant.
Of a total of 62 patients assessed for eligibility, 56 subjects were randomized into two groups and 4 patients were excluded from the study due to failed puncture (n=1) and prolonged surgery (n=3) (Fig. 2). Consequently, 52 patients completed the study.
Patient characteristics were comparable between the two groups (Table 1). Forty of the 52 patients (76.9%) had more than one co-existing disease. MAP and HR were stable during the procedure in both groups. No significant differences were observed in MAP and HR between the two groups at each time point (Fig. 3).
Spinal anesthetic characteristics are presented in Table 2. Cold, sensory, and touch peak block levels were not different between the two groups. Time to peak block was similar in the two groups. In both groups, the cold block level was 1 segment higher than pinprick block level, but there was no significant difference between the two block levels. In comparison, the level to touch sense loss was significantly lower than cold or pinprick block in both groups (p=0.001) (Table 2). And 23 patients (88.5%) of Group F (n=26) and 20 patients (76.9%) of Group S (n=26) scored 5 on the motor block scale, and the rest of the patients scored 4 at the time of peak block.
Two patients (7.7%) in each group were given supplemental fentanyl 50 µg or sufentanil 10 µg intraoperatively (Table 2). Time to regression of two sensory dermatomes was significantly longer in Group S than Group F (p=0.017). The motor block scale score at that moment was a 5 in all patients. Table 3 lists postoperative characteristics. In Group S, the postoperative analgesic requirement was less and the time to the first analgesic request was longer than that for Group F (p=0.023, p=0.025). Nausea or vomiting did not occur in any patients. On the other hand, pruritus and PDPH occurred in 3 and 1 patients in Group S, respectively. And postoperative pain scores were significantly lower in Group S than Group F at 6 (p=0.003), 12 (p=0.014), and 18 hours postoperatively (p=0.034) (Fig. 4). The satisfaction levels of both groups were similar (92.3% reported good satisfaction in each group).
As the demand of one-day surgery has increased, the demand for the anesthetic techniques that can provide fast recovery with stable hemodynamics has also increased. In elderly patients requiring spinal anesthesia, restricted block with a low-dose of local anesthetic is desirable. In the current study, SSA using 1 mg of bupivacaine combined with fentanyl 20 µg or sufentanil 5 µg provided sufficient anesthesia for TURP. Postoperative analgesic requirement was less in Group S (7.7%) than in Group F (34.6%).
Conventionally, 10-12.5 mg of intrathecal bupivacaine is administered to obtain a sensory block up to T10 for TURP. However, such doses frequently induce high sympathetic block, cardiovascular instability, and intense motor block in elderly patients.15,18,24 In the present study, 76.9% of patients had more than one systemic disease, which is comparable to the results of previous studies.3,4,5 Since a decrease in MAP after spinal block is correlated with sympathetic block, it is important to restrict the block level in elderly patients. As mentioned earlier, an analgesia level up to T12 could provide sufficient anesthesia for TURP considering the pain pathway and sympathetic block level.10
For spinal block in short transurethral procedures, 3 mg of bupivacaine combined with fentanyl 20 µg, used in a study by Zohar, et al.,25 was the lowest dose ever reported. Through a dose-response study prior to the present study, we found that even 1 mg of bupivacaine combined with fentanyl or sufentanil provides sufficient sensory block while sparing motor function. Thus, we thought it worthy to investigate the effectiveness of 1 mg of bupivacaine with opioid in spinal block for elderly patients undergoing TURP.
In our present study, the median sensory block levels were comparable between both groups (Group F, T12 vs. Group S, T11). Even though the block range varied (Group F, S1-T6 vs. Group S, L4-T6), every surgery was completed successfully within 1 hour. However, this extremely small dose may not be appropriate for large prostates or other types of surgery. Compared to a previous study,4 peak sensory block levels were similar and stable hemodynamics persisted in both studies. In this current study, however, time to sensory regression and postoperative analgesia were shorter, and motor function was more preserved. In the previous study,4 48.6% of patients showed a motor block scale score <4 (able to raise their legs, but unable to keep them raised) at peak sensory block, and 8.5% of patients still showed a motor block scale score <4 at the time of two sensory dermatomes regression. Nevertheless, all patients moved themselves to the bed without aid when they arrived at the admission room in this study. Spared motor function allows for more rapid discharge and increases patient satisfaction. Patients who showed "good" satisfaction commented that spared motor function made them feel more comfortable. Ten of 52 patients who had experienced spinal anesthesia before were more satisfied with this method. Even though impaired functional motor balance may remain long after motor power recovery in spinal anesthesia,26 this could be negligible with ultralow-dose bupivacaine.
Group S demonstrated a longer time to sensory regression and better postoperative analgesia than Group F (Table 2 and 3). These characteristics may be related to the density of the drug solution and physiochemical properties such as dose, lipid solubility, and affinity to the opioid µ receptor. The density of the drug solution could be a major factor affecting the intrathecal spread of a drug.26 In a spinal model, intrathecal anesthetic spread was found to be influenced by a density difference as small as 0.0006 mg/mL.27 Although the densities of fentanyl and sufentanil are similar,27 the density of the sufentanil-mixed solution that we used was higher than the fentanyl-mixed solution (1.0182±0.0006 vs. 1.0172±0.0003). This difference could be a factor for the different anesthetic characteristics between two groups. Also, we selected the doses of fentanyl (20 µg) and sufentanil (5 µg) to compare the efficacy. However, we observed a little greater proportion of sufentanil than a dose ratio of 4.4:1, which was reported as an equipotent dose ratio of fentanyl and sufentanil.28 Thus, this difference in dose ratio could be a factor in prolonged postoperative analgesia in Group S. Sufentanil is 8-10 times more lipid-soluble than fentanyl, however, the difference in lipid solubility may not be an important factor for intrathecal action because the drugs have more direct access to the spinal nerves through the cerebrospinal fluid.28 Also, the high affinity of sufentanil to the µ-opioid receptor in the spinal cord may increase the duration of the sensory block while preserving motor function.29
There are some limitations in this study. We calculated the sample size with expectation of different sensory block level between two groups, based on our previous study.4 However, peak sensory block levels were similar in both groups. Thus, the sample size might not be appropriate for this study. Secondly, we do not think that this study can be applicable to every TURP: the characteristics of different ethnic groups may affect the generalizability of the results. Based on the relationship between ethnic groups and µ-opioid receptor genes, the mutant variant is more sensitive and prevalent in Asian populations, therefore, lower doses may be required in Asians.30 Furthermore, since the spinal length from C7 to the sacral hiatus would be important in intrathecal drug spread,31 we cannot exclude the influence of height on drug spread in this current study: Westerners are generally taller than Asians. Thus, the block level with an extremely low-dose of bupivacaine may be different between Asians and Caucasians.
In conclusion, SSA using 1 mg of bupivacaine with fentanyl or sufentanil may be useful for TURP in elderly patients, especially for ambulatory cases. For this SSA, anesthesiologists and urologists need to communicate with each other to maintain lower bladder distension pressure. However, this ultralow dose may not be appropriate for other types of surgery.
Figures and Tables
Fig. 1
Consecutive dose of bupivacaine for successful spinal block in fentanyl group and sufentanil group. White circle, successful block; black circle, insufficient block. F, fentanyl; S, sufentanil.

Fig. 3
Mean arterial pressure and heart rate changes during procedure. F, fentanyl; S, sufentanil; MAP, mean arterial pressure; HR, heart rate.

Fig. 4
Verbal numerical rating scales (vNRS) for pain during 36 hours after surgery. Box plot with median (solid line), interquartile range (box), and values within 1.5 times the interquartile range (whiskers). Outliers are indicated by circles. *p<0.05 compared with the Group F. F, fentanyl; S, sufentanil.

Table 1
Patient Characteristics

Table 2
Anesthetic Characteristics
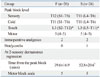
F, fentanyl; S, sufentanil.
Values are mean±SD, median (range) or number. Motor block scale: 1, complete motor block; 2, able to move feet only; 3, able to move feet and bend knees; 4, able to straight leg raise <30°; 5, able to straight leg raise >30°.
*p<0.001 compared with cold or sensory within each group.
†p<0.05 compared with Group F.
ACKNOWLEDGEMENTS
The result of this study was presented at the annual meeting of American Society of Anesthesiology on Oct. 2012.
This study was partly supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, and Republic of Korea (A084120).
References
1. Arrighi HM, Metter EJ, Guess HA, Fozzard JL. Natural history of benign prostatic hyperplasia and risk of prostatectomy. The Baltimore Longitudinal Study of Aging. Urology. 1991; 38:1 Suppl. 4–8.

2. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984; 132:474–479.

3. Li MK, Garcia L, Patron N, Moh LC, Sundram M, Leungwattanakij S, et al. An Asian multinational prospective observational registry of patients with benign prostatic hyperplasia, with a focus on comorbidities, lower urinary tract symptoms and sexual function. BJU Int. 2008; 101:197–202.

4. Kim SY, Cho JE, Hong JY, Koo BN, Kim JM, Kil HK. Comparison of intrathecal fentanyl and sufentanil in low-dose dilute bupivacaine spinal anaesthesia for transurethral prostatectomy. Br J Anaesth. 2009; 103:750–754.

5. Hong JY, Yang SC, Ahn S, Kil HK. Preoperative comorbidities and relationship of comorbidities with postoperative complications in patients undergoing transurethral prostate resection. J Urol. 2011; 185:1374–1378.

6. Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. J Urol. 2008; 180:246–249.

7. Vaghadia H, Neilson G, Lennox PH. Selective spinal anesthesia for outpatient transurethral prostatectomy (TURP): randomized controlled comparison of chloroprocaine with lidocaine. Acta Anaesthesiol Scand. 2012; 56:217–223.

8. Malhotra V, Sudheendra V, O'Hara J, Diwan S. Anesthesia and the renal and genitourinary systems. In : Miller RD, editor. Miller's anesthesia. Philadelphia: Churchill Livingstone/Elsevier;2010. p. 2105–2134.
9. Chung BI, Sommer G, Brooks JD. Anatomy of the lower urinary tract and male genitalia. In : Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10th ed. Philadelphia, PA: Saunders/Elsevier;2012. p. 33–70.
10. Beers RA, Kane PB, Nsouli I, Krauss D. Does a mid-lumbar block level provide adequate anaesthesia for transurethral prostatectomy? Can J Anaesth. 1994; 41:807–812.

11. Racle JP, Benkhadra A, Poy JY, Gleizal B. Spinal analgesia with hyperbaric bupivacaine: influence of age. Br J Anaesth. 1988; 60:508–514.

12. Carpenter RL, Caplan RA, Brown DL, Stephenson C, Wu R. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992; 76:906–916.

13. Rocco AG, Raymond SA, Murray E, Dhingra U, Freiberger D. Differential spread of blockade of touch, cold, and pinprick during spinal anesthesia. Anesth Analg. 1985; 64:917–923.

14. Chamberlain DP, Chamberlain BD. Changes in the skin temperature of the trunk and their relationship to sympathetic blockade during spinal anesthesia. Anesthesiology. 1986; 65:139–143.

15. Vaghadia H. Spinal anaesthesia for outpatients: controversies and new techniques. Can J Anaesth. 1998; 45(5 Pt 2):R64–R75.

16. Fleisher LA, Mantha S, Roizen MF. Medical technology assessment: an overview. Anesth Analg. 1998; 87:1271–1282.
17. Wassef MR, Michaels EI, Rangel JM, Tsyrlin AT. Spinal perianal block: a prospective, randomized, double-blind comparison with spinal saddle block. Anesth Analg. 2007; 104:1594–1596.

18. Carron M, Freo U, Veronese S, Innocente F, Ori C. Spinal block with 1.5 mg hyperbaric bupivacaine: not successful for everyone. Anesth Analg. 2007; 105:1515–1516.

19. Kararmaz A, Kaya S, Turhanoglu S, Ozyilmaz MA. Low-dose bupivacaine-fentanyl spinal anaesthesia for transurethral prostatectomy. Anaesthesia. 2003; 58:526–530.

20. Ben-David B, Solomon E, Levin H, Admoni H, Goldik Z. Intrathecal fentanyl with small-dose dilute bupivacaine: better anesthesia without prolonging recovery. Anesth Analg. 1997; 85:560–565.
21. Hamber EA, Viscomi CM. Intrathecal lipophilic opioids as adjuncts to surgical spinal anesthesia. Reg Anesth Pain Med. 1999; 24:255–263.

22. de Santiago J, Santos-Yglesias J, Giron J, Montes de Oca F, Jimenez A, Diaz P. Low-dose 3 mg levobupivacaine plus 10 microg fentanyl selective spinal anesthesia for gynecological outpatient laparoscopy. Anesth Analg. 2009; 109:1456–1461.

23. Imarengiaye CO, Song D, Prabhu AJ, Chung F. Spinal anesthesia: functional balance is impaired after clinical recovery. Anesthesiology. 2003; 98:511–515.
24. Kuusniemi KS, Pihlajamäki KK, Pitkänen MT, Helenius HY, Kirvelä OA. The use of bupivacaine and fentanyl for spinal anesthesia for urologic surgery. Anesth Analg. 2000; 91:1452–1456.

25. Zohar E, Noga Y, Rislick U, Leibovitch I, Fredman B. Intrathecal anesthesia for elderly patients undergoing short transurethral procedures: a dose-finding study. Anesth Analg. 2007; 104:552–554.

26. Richardson MG, Wissler RN. Densities of dextrose-free intrathecal local anesthetics, opioids, and combinations measured at 37 degrees C. Anesth Analg. 1997; 84:95–99.

27. Stienstra R, Gielen M, Kroon JW, Van Poorten F. The influence of temperature and speed of injection on the distribution of a solution containing bupivacaine and methylene blue in a spinal canal model. Reg Anesth. 1990; 15:6–11.
28. Nelson KE, Rauch T, Terebuh V, D'Angelo R. A comparison of intrathecal fentanyl and sufentanil for labor analgesia. Anesthesiology. 2002; 96:1070–1073.

29. Standl TG, Horn E, Luckmann M, Burmeister M, Wilhelm S, Schulte am Esch J. Subarachnoid sufentanil for early postoperative pain management in orthopedic patients: a placebo-controlled, double-blind study using spinal microcatheters. Anesthesiology. 2001; 94:230–238.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download