Abstract
Purpose
This study attempted to derive an objective and sophisticated definition of poor ovarian response (POR).
Materials and Methods
A total of 176 consecutive in vitro fertilization (IVF) cycles (137 patients) with conventional ovarian stimulation during 2009 to 2012 were studied by retrospective analysis. Optimal oocyte number (total or mature) was determined by statistics-based (distribution of oocyte number) and prognosis-based approaches (prediction for IVF outcome). Receiver operating characteristics curve analysis was used to show what number of oocytes could predict IVF pregnancy and whether clinical and laboratory variables could predict newly defined POR.
Results
The 25th percentile of the distribution corresponded to total oocytes ≤2 and mature oocyte ≤1. The cut-off values for the prediction of IVF outcomes were total oocytes >5 and mature oocyte >1. Considering the incidence of POR (34.1%), a reasonable definition of POR was decided as total oocytes ≤2 or mature oocyte ≤1. For the prediction of this new definition, the extreme cut-off value (by setting a false positive rate of 5%) of serum anti-Mullerian hormone (AMH) was ≤0.76 ng/mL, which was better than serum follicle stimulating hormone or age. A new simple definition of POR was derived as total oocytes ≤2 or mature oocyte ≤1 in a previous cycle or a serum AMH level of ≤0.76 ng/mL. When this simple criterion was re-applied to our data, the predictive performance was similar to the Bologna criteria.
Retrieval of healthy oocytes is crucial to in vitro fertilization (IVF) treatment for infertile couples. Although mild ovarian stimulation has been introduced, most infertility centers still use conventional ovarian stimulation, which is defined as obtaining more than eight oocytes. Sometimes, however, only a few oocytes are obtained, leading to total fertilization failure or no transferrable embryos. This unsatisfactory circumstance, usually termed as poor ovarian response (POR), has a psychological impact and places an economic burden on infertile couples.
An enormous number of published studies have addressed the pathogenesis, prediction, and possible treatments of POR; however, a uniform definition of POR has not yet been agreed upon.1,2,3,4 A systemic review of 47 randomized trials showed that 41 different definitions were used for poor ovarian response.3 With the lack of a uniform definition, researchers are unable to effectively estimate and compare the incidences thereof. Accordingly, the European Society of Human Reproduction and Embryology (ESHRE) working group proposed the Bologna criteria for defining POR to ovarian stimulation for IVF in 2011.5
According to the Bologna criteria, at least two of the following three features must be present: 1) advanced maternal age (≥40 years) or any other risk factor for POR; 2) a previous POR (≤3 oocytes with a conventional ovarian stimulation protocol); or 3) an abnormal ovarian reserve test (ORT), antral follicle count (AFC) <5-7 or serum anti-Mullerian hormone (AMH) <0.5-1.1 ng/mL. Those with advanced maternal age or abnormal ORT are to be classified as "expected POR." Therefore, the most important component in the Bologna criteria is a previous POR.
In order to reach a common and universal definition, all components thereof should be selected based on scientific evidence. Although the Bologna criteria have emerged as a definition for POR,6,7 several criticisms have arisen against the criteria: first, patients included in their study were very diverse.8 Second, although maternal age and previous POR were well defined, detailed risk factors for POR were not described.5 Third, the cut-off levels of ORT were not suggested with clear cut-off points for each ORT included, especially in the case of AMH; the wide range of the cut-off levels does not offer uniform criteria. Lastly, the Bologna criteria were not based on scientific experiments and were just combined from criteria reported in previous studies. In fact, POR was defined as an oocyte number of less than four (irrespective of oocyte maturity) in the Bologna criteria since this number is most often used in the literature. However, defining the number of oocytes for POR should be made on scientific and epidemiologic bases. The term POR should be determined based on IVF outcomes [e.g., a significant decrease in clinical pregnancy rate (CPR) and live birth rate (LBR)].9
Two approaches can be utilized for defining an appropriate oocyte number for POR. These include statistical and prognosis-based approaches. One common approach to defining a certain variable as "abnormal" is to define it as less than the 5th or 10th percentile among a normal distribution. In such statistical approaches, cut-off values should be interpreted on the basis of disease prevalence, and for POR, its prevalence may vary according to a women's age. Meanwhile, as POR reflects a poor prognosis, the likelihood of it occurring can be determined by assessing treatment outcomes (i.e., clinical pregnancy, ongoing pregnancy, or live birth).
Here, we verified the appropriateness of the Bologna criteria and developed a more sophisticated definition by assessing oocyte number for defining POR based on statistical and prognosis-based approaches. We also evaluated the diagnostic performance of various factors in predicting POR according to our new definition. Finally, both the Bologna criteria and our new definition of POR were adapted to our data in which the incidence of POR, as well as diagnostic and clinical performance, was assessed.
A retrospective study was performed including 176 consecutive IVF/intracytoplasmic sperm injection (ICSI) cycles (137 patients) with conventional ovarian stimulation during 2009 to 2012 at a university-based hospital. The eligible cohort comprised all consecutive patients who fulfilled the following criteria: 1) having at least 1 year of infertility, 2) being treated with a long agonist or antagonist protocol regimen (mostly GnRH antagonist protocol), and 3) having AMH values tested in our laboratory during the preliminary fertility workup at their first consultation. Cycles with mild stimulation protocol and cycles with total gonadotropin less than 500 IU were excluded. The study was approved by the Institutional Review Board of Seoul National University Bundang Hospital.
Several numerical [age, body mass index (BMI), number of present children, number of previous IVF cycles, cycle number, serum AMH, serum follicle stimulating hormone (FSH), total gonadotropin dose administered, number of total and mature oocytes, and number of embryos transferred] and categorical variables (infertility diagnosis, previous ovarian surgery, having one ovary) were recorded. Blood samples were taken from the cubital vein in the early follicular phase (day 2 or day 3), before any IVF-related drug administration. Serum AMH was measured by enzyme-linked immunosorbent assay (ELISA) using the Beckman Coulter AMH ELISA kit (Immunotech, Marseilles, France). The basic IVF and embryo transfer (ET) protocol was performed as described previously.10 Clinical pregnancy was defined as visualization of a gestational sac on ultrasound with evidence of biochemical pregnancies. Live birth was defined as the birth of at least one live-born child. The basic clinical characteristics of the patients and IVF cycles are shown in Table 1.
Medcalc version 12.6 (Medcalc Software, Mariakerke, Belgium) was used for statistical analysis and the results were considered statistically significant at p-values of <0.05. The chi-squared test was used to compare proportions between two groups. Univariable and multivariable regression analyses were performed for several variables [AMH, FSH, age, BMI (kg/m2), number of previous IVF cycles, number of living child, total FSH administered]. Receiver operating characteristics (ROC) curve analysis was performed to show whether the numerical variables can predict outcomes. Finally cut-off levels of AMH and number of retrieved oocytes for defining poor ovarian response were obtained using ROC curves.
We performed exploratory data analysis for number of total or mature oocytes. The number of retrieved oocytes did not show a normal distribution (Fig. 1). The median numbers were 5 (range 0 to 34) for total oocytes and 3 (range 0 to 20) for mature oocytes. The skewness was 1.5 for total oocytes and 1.7 for mature oocytes. The 5th and 10th percentiles were 0.3 and 1 for total oocytes and 0 and 0 for mature oocytes, respectively. When POR was defined as total oocytes ≤2 (25th percentile), the incidence of POR was 25.6% (45/176), and the pregnancy rate (PR)/ET in the POR group was 22.2% (6/27), which was lower than PR/ET for the non-POR group (41.7%, 53/127, p=0.058); 40% of the POR cycles did not enter ET. When POR was defined as mature oocytes ≤1, the incidence of POR was 31.3% (55/176) and the PR/ET in the POR group was 16.7% (6/36), which was significantly lower than PR/ET for the non-POR group (44.9%, 53/118, p=0.002); the percentage of POR cycles not entering ET was 34.5%.
We analyzed cut-off values of oocyte number using ROC curves to predict IVF outcomes. Interestingly, all cut-off values for oocyte number were the same for clinical or ongoing pregnancy and live birth (i.e., total oocytes >5 and mature oocytes >1) (Table 2). Taken together, our findings from both statistical and prognosis-based approaches supported 'mature oocytes ≤1' as a definition of POR. The choice of 'total oocytes ≤5' as a definition of POR might be unrealistic as the incidence of POR would be too high (50.6%).
Considering both the statistical and prognosis-based approaches, we speculated that it would be reasonable to choose total oocytes ≤2 or mature oocytes ≤1 as the definition of POR. When POR was defined as 'total oocytes ≤2' or 'mature oocyte ≤1', the incidence of POR was 34.1% (60/176) and the PR/ET in the POR group was 19.5% (8/41), which was significantly lower than PR/ET in the non-POR group (45.1%, 51/113, p=0.004). The percentage of POR cycles not entering ET was 31.7%.
In univariable and multivariable regression analysis of the numerical and categorical variables listed in the methods, serum AMH, serum FSH, and age were shown to be related with total oocyte number and mature oocyte number (data not shown).
ROC curve analysis showed the cut-off values when POR was defined as total oocytes ≤2 or mature oocyte ≤1. For the purpose of screening method, the cut-off values of any predictor might be determined as those with low false positive rate, even though this reduces sensitivity. Therefore, we also presented the extreme cut-off values by setting a false positive rate of 5% (i.e., specificity 95%) (Table 3). The predictive performance of serum AMH was superior to both serum FSH, and age, although the differences therein were not statistically significant (Fig. 2). Combining the serum FSH or age did not enhance the predictive performance significantly (AUC 0.787 vs. AUC 0.823, p=0.246).
Considering the best predictive performance of serum AMH with low false positive rate, we propose a new definition of POR: POR could be defined as total oocytes ≤2 or mature oocytes ≤1 obtained in a previous cycle or when serum AMH ≤0.76 ng/mL.
When these simple criteria were re-applied to our data, the incidence of predicted POR was 20.5% (36/176), with a sensitivity of 45.0%, specificity of 92.2%, positive predictive value of 75.0%, and negative predictive value of 76.4% (Table 4). Compared with the Bologna criteria, our new definition showed a similar predictive performance.
Poor ovarian response limits IVF success, and assessing various interventions is difficult because of wide variations in defining POR. In 2011, the ESHRE working group proposed the Bologna criteria to define POR to ovarian stimulation for IVF. However, as they stated, the aim of the Bologna criteria was to identify POR only for research purposes and that the criteria have no absolute value in predicting prognosis.4 The goal of ORT, especially in POR patients, is to add more prognostic information to the counseling and planning process in order to help couples choose among treatment options and to have a realistic expectations of fecundity. Thus, we attempted to develop a more objective and scientific definition of POR. In doing so, we discerned that POR could be defined as total oocytes ≤2 or mature oocytes ≤1 obtained in a previous cycle or for serum AMH ≤0.76 ng/mL (as an expected POR).
Sallam, et al.11 also attempted to define POR on the basis of IVF prognosis. In their study, CPR started to become significantly lower when fewer than five, six, and eight (maybe total) oocytes were retrieved from patients treated with ICSI, conventional IVF, or TESE/ICSI, respectively. Also, McAvey, et al.12 evaluated the association between the number of mature oocytes per IVF cycle and the likelihood of live birth. In conclusion there was an advantage to obtaining six or more mature oocytes during the fresh oocyte retrieval, compared with five or fewer oocytes. However, the choice of such a high oocyte number as the definition of POR might be impractical, as the incidence of POR would be too high.
In the Bologna criteria, AFC and AMH were used as ORT. However, AFC was not analyzed in the present study because objective reporting by the same observer was not available for all patients. Although the diagnostic performance of AFC in the prediction of poor ovarian response is good, recent literature indicates a new hormonal marker, AMH, as a preferred marker.13,14 The Practice Committee of the American Society for Reproductive Medicine concluded that there is mounting evidence to support the use of AMH as a screening test for POR and insufficient evidence to support the use of AFC as a screening test for failure to conceive.4 Among the numerical and categorical variables analyzed in the present study, only three variables (serum AMH, serum FSH, and age of women) could predict the POR with a statistical significance. Therefore, several risk factors proposed by the Bologna criteria were not included in our new definition. Indeed, 21 women in our series received ovarian surgery; however, ovarian response and IVF outcomes were similar between women with or without previous ovarian surgery.
ROC curves provide a graphic representation of a tradeoff between sensitivity and specificity. One of the commonly used criterion is Youden index that maximizes the sum of sensitivity and specificity. The cut-point leading to the index is the optimal cut-point when equal weight is given to sensitivity and specificity. It both measures the effectiveness of a diagnostic marker and enables the selection of an optimal threshold value (cutoff point) for the marker. The values for AMH, age, and FSH that resulted in maximizing the Youden index were defined as "usual cut-off values." However, in the context of defining POR, disease positive indicates individuals with poor response and disease negative indicates those with a normal or high response. A false positive refers to good responders, although it is wrongfully assigned to poor responders. From a practical viewpoint, false positive rates should be lower than 5%. This is, in general, a prerequisite for proper screening tests. Thus, in our study, "usual cut-off values" were derived from the Youden index, while the maximal deflection points and "extreme cut-off values" were chosen at the point at which the false positive rate was 5%.
In the present study, when POR was defined as total oocytes ≤2 or mature oocyte ≤1, the AUC of serum AMH was highest and combining serum FSH or age did not enhance the predictive performance thereof significantly. Currently, there is mounting evidence of serum AMH as a better predictor of POR, compared to serum FSH or age of a woman.15-24 This finding was subsequently confirmed in an individualized patient data meta-analysis, with no significant improvement in classification of a poor ovarian response, above a basal AMH when age, FSH, or AFC were also taken into account. Considering these findings, serum AMH appears to be an optimal variable for defining POR. Additionally, a previous meta-analysis concluded that basal FSH is not a useful predictor of IVF outcome,25 possibly because of inter-cycle variability.26 Meanwhile, serum AMH exhibits little inter-cycle variability in comparison to serum FSH; it is well-known as a quantitative indicator of oocyte number and as a qualitative indicator of oocyte quality and clinical pregnancy.21,27,28 Although several studies have recently shown that serum AMH is affected by a number of factors, including obesity, serum vitamin D, and serum leptin,29,30,31,32 AMH remains one of the most reliable markers of ovarian reserve. Therefore, we proposed that serum AMH alone is sufficient as a predictor of expected POR. Nevertheless, studies have suggested that AMH <0.1-2.0 ng/mL is a poor predictor of ovarian response and likely CPR in all women with normal ovarian reserve.15,16,17,18,19,20,21,22,23,24 Moreover, an AMH <0.2 ng/mL has been suggested as a poor predictor of CPR in women with POR.33,34 On the other hand, Gleicher, et al.35 showed that AMH ≥1.06 ng/mL is a predictor of good LBR in women with POR.
When we applied the Bologna criteria or our new definition to our data, the predictive performances were similar. The incidence of expected POR was higher when our new definition was applied (27 vs. 36), although the incidences of true POR were the same for both criteria. Applying either the Bologna criteria or our new definition applied, expected POR had a similar ongoing PR: 11.1%/cycle (3/27) and 17.6%/ET (3/17) vs. 13.9%/cycle (5/36), and 20.0%/ET (5/25).
Although limited by the relatively small sample size, we propose a new definition of POR, which is simple and based on statistical and prognosis-based evidence. We adopted serum AMH alone as a predictor for greater simplicity of the prediction model and adopted a lower false positive rate for serum AMH as a prerequisite of an appropriate screening method. Our new definition might be helpful when selecting patients in clinical practice, as well as for research purposes.
Figures and Tables
Fig. 1
Distribution of number of retrieved oocytes. (A) Total oocyte. (B) Mature oocyte. Each percentile value is shown with lines.

Fig. 2
Pairwise comparison of three ROC curves. Each ROC curve was constructed to predict POR defined as total oocytes ≤2 or mature oocyte ≤1. Area under the curve of serum AMH was highest (AMH 0.787, FSH 0.712, age 0.730). ROC, receiver operating characteristic; FSH, follicle stimulating hormone; POR, poor overian response; AMH, anti-Mullerian hormone.

Table 1
Basic Clinical Characteristics of the Patients and IVF Cycles

Table 2
Cut-Off Values of Oocyte Number to Predict IVF Outcomes

Table 3
Predictive Performance of Serum AMH, FSH, and Age of Woman to Predict POR Defined as Total Oocytes ≤2 or Mature Oocyte ≤1
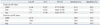
Table 4
Application of the Bologna Criteria or Our New Definition to Our Data
ACKNOWLEDGEMENTS
This work was supported by grant no. A120043 from the Korea Health Care Technology R&D Project, Ministry of Health and Welfare, Korea.
References
1. Pandian Z, McTavish AR, Aucott L, Hamilton MP, Bhattacharya S. Interventions for 'poor responders' to controlled ovarian hyper stimulation (COH) in in-vitro fertilisation (IVF). Cochrane Database Syst Rev. 2010; 20:CD004379.

3. Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. 2011; 96:1058–1061.
4. Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2012; 98:1407–1415.
5. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011; 26:1616–1624.

6. Polyzos NP, Tournaye H, Devroey P. AMH for predicting poor ovarian responders in GnRH antagonist cycles. Hum Reprod. 2012; 27:1876–1877.

7. Polyzos NP, De Vos M, Corona R, Vloeberghs V, Ortega-Hrepich C, Stoop D, et al. Addition of highly purified HMG after corifollitropin alfa in antagonist-treated poor ovarian responders: a pilot study. Hum Reprod. 2013; 28:1254–1260.

8. Younis JS. The Bologna criteria for poor ovarian response; has the job been accomplished? Hum Reprod. 2012; 27:1874–1875.

9. Sallam HN, Ezzeldin F, Agameya AF, Abdel-Rahman AF, El-Garem Y. The definition of 'poor response': Bologna criteria. Hum Reprod. 2012; 27:626–627.

10. Lee JE, Lee JR, Jee BC, Suh CS, Kim KC, Lee WD, et al. Clinical application of anti-Müllerian hormone as a predictor of controlled ovarian hyperstimulation outcome. Clin Exp Reprod Med. 2012; 39:176–181.

11. Sallam HN, Ezzeldin F, Agameya AF, Rahman AF, El-Garem Y. Defining poor responders in assisted reproduction. Int J Fertil Womens Med. 2005; 50:115–120.
12. McAvey B, Zapantis A, Jindal SK, Lieman HJ, Polotsky AJ. How many eggs are needed to produce an assisted reproductive technology baby: is more always better? Fertil Steril. 2011; 96:332–335.

13. La Marca A, Argento C, Sighinolfi G, Grisendi V, Carbone M, D'Ippolito G, et al. Possibilities and limits of ovarian reserve testing in ART. Curr Pharm Biotechnol. 2012; 13:398–408.

14. Ledger WL. Clinical utility of measurement of anti-mullerian hormone in reproductive endocrinology. J Clin Endocrinol Metab. 2010; 95:5144–5154.

15. van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002; 17:3065–3071.
16. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles--implications for individualization of therapy. Hum Reprod. 2007; 22:2414–2421.

17. Muttukrishna S, Suharjono H, McGarrigle H, Sathanandan M. Inhibin B and anti-Mullerian hormone: markers of ovarian response in IVF/ICSI patients? BJOG. 2004; 111:1248–1253.

18. Kwee J, Schats R, McDonnell J, Themmen A, de Jong F, Lambalk C. Evaluation of anti-Müllerian hormone as a test for the prediction of ovarian reserve. Fertil Steril. 2008; 90:737–743.

19. La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, Levratti P, et al. Anti-Müllerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod. 2004; 19:2738–2741.

20. Fiçicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimüllerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006; 85:592–596.

21. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006; 21:2022–2026.

22. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimüllerian hormone/müllerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004; 82:1323–1329.

23. Elgindy EA, El-Haieg DO, El-Sebaey A. Anti-Müllerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril. 2008; 89:1670–1676.

24. Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008; 23:1359–1365.

25. Bancsi LF, Broekmans FJ, Mol BW, Habbema JD, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003; 79:1091–1100.

26. Bancsi LF, Broekmans FJ, Looman CW, Habbema JD, te Velde ER. Predicting poor ovarian response in IVF: use of repeat basal FSH measurement. J Reprod Med. 2004; 49:187–194.
27. Buyuk E, Seifer DB, Younger J, Grazi RV, Lieman H. Random anti-Müllerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011; 95:2369–2372.

28. Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006; 21:159–163.

29. Buyuk E, Seifer DB, Illions E, Grazi RV, Lieman H. Elevated body mass index is associated with lower serum anti-mullerian hormone levels in infertile women with diminished ovarian reserve but not with normal ovarian reserve. Fertil Steril. 2011; 95:2364–2368.

30. Merhi ZO, Minkoff H, Feldman J, Macura J, Rodriguez C, Seifer DB. Relationship of bariatric surgery to Müllerian-inhibiting substance levels. Fertil Steril. 2008; 90:221–224.

31. Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab. 2012; 97:2450–2455.

32. Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua S Jr, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013; 28:1661–1669.

33. Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010; 93:855–864.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download