Abstract
Purpose
The aim of this study was to identify risk factors influencing permanent stomas after low anterior resection with temporary stomas for rectal cancer.
Materials and Methods
A total of 2528 consecutive rectal cancer patients who had undergone low anterior resection were retrospectively reviewed. Risk factors for permanent stomas were evaluated among these patients.
Results
Among 2528 cases of rectal cancer, a total of 231 patients had a temporary diverting stoma. Among these cases, 217 (93.9%) received a stoma reversal. The median period between primary surgery and stoma reversal was 7.5 months. The temporary and permanent stoma groups consisted of 203 and 28 patients, respectively. Multivariate analysis showed that independent risk factors for permanent stomas were anastomotic-related complications (p=0.001) and local recurrence (p=0.001). The 5-year overall survival for the temporary and permanent stoma groups were 87.0% and 70.5%, respectively (p<0.001).
The development of mechanical circular stapler devices and total mesorectal excision with preoperative chemoradiation for the treatment of rectal cancer has allowed for sphincter-saving surgeries, improved oncologic outcomes, and also decreased the prevalence of requiring a permanent stoma.1,2,3,4 Anastomotic leakage after sphincter-saving surgery is the most worrisome complication of stoma creation, as it associated with numerous unfavorable clinical outcomes including postoperative morbidity and early mortality.5,6 Diverting stomas can minimize the fatal consequences of anastomotic leakage, although they do not substantially decrease its incidence.7,8,9 While diverting stomas are intended to be temporary, they are also associated with permanent stomas, specifically non-reversal of the protective stoma and secondary construction of a permanent stoma. Several recent studies have shown that risk factors for permanent stomas include old age, anastomotic-related complications, local recurrence, radiotherapy, and poor anal function; however, these risk factors are not well established.10,11,12,13 In addition, there have been few reports focusing on risk factors of permanent stomas after creating a primary diverting stoma.
The aim of this study was to identify the risk factors influencing the need for a permanent stoma after low anterior resection with a temporary stoma for rectal cancer.
We retrospectively reviewed a total of 2528 consecutive primary rectal cancer patients with histologically confirmed adenocarcinoma who underwent low anterior resection with temporary stomas from January 2000 to December 2009 at our institution. Patients who underwent palliative resection or emergency surgery or had recurrent cancer or distant metastasis were excluded from this study. In addition, patients who died within 90 days after primary surgery were excluded. Ultimately, a total of 231 patients were enrolled in our analysis (Fig. 1) and further divided into two groups: a temporary stoma group and a permanent stoma group. After placing patients into the two groups, the risk factors for permanent stoma were evaluated. Permanent stomas included stoma re-creation after reversal and stoma non-reversal after diversion. This study was approved by the Institutional Review Board of Samsung Medical Center, and all patients gave informed consent.
Rectal cancer was determined by sigmoidoscopy and defined as a tumor with a lower border within 15 cm of the anal verge. Anastomosis-related complications included anastomotic leakage, stricture, fistula, and pelvic abscess. Anastomotic leakage was defined as clinical signs of peritonitis and radiological findings of extraluminal air, fistula, or intra-abdominal abscess. Patients with radiologically demonstrated anastomotic leakage without clinical evidence were excluded. Anorectal stricture was defined as stenosis requiring management either manually or with a Hegar dilator. Early anastomotic-related complications were defined as those occurring within 90 days of the operation, while all others were considered late complications.
Patients were followed up at 3-month intervals for the first 2 years after surgery and then every 6 months for 5 years. Colon enema with water-soluble contrast media and colonoscopy were performed to evaluate any abnormalities such as leakage or stricture. If abnormal findings were observed, stoma closure was delayed until the leakage had healed or the stricture had improved by dilatation.
Statistical analyses were performed with SPSS (version 19; SPSS Inc., Chicago, IL, USA). The χ2 test or Fisher's exact were used for univariate analyses for risk factors of permanent stomas. Independent risk factors were calculated by multivariate analysis with logistic regression. Only parameters with values of p<0.20 were included in multivariate analysis. The overall survival rate was analyzed with the Kaplan-Meier method and the log-rank test. p-values <0.05 were considered statically significant.
There were 231 patients included in our study, and 217 (93.9%) received a stoma reversal. The median period between primary surgery and stoma reversal was 7.5 months. After a median follow-up of 50.4 months (range 4-156 months), 203 patients were confirmed to have had a temporary stoma while 28 patients required a permanent stoma. Of the 28 permanent stoma patients (12.1%), there were 14 ileostomies (50.0%), eight colostomies (28.6%), and four abdominoperineal resections (14.3%), as well as one patient (3.6%) who underwent Hartmann's operation.
The characteristics of the temporary and permanent stoma groups are shown in Table 1. The risk of requiring a permanent stoma included anastomotic-related complications (p=0.001) and local recurrence (p=0.001). Multivariate analysis showed that the independent risk factors for permanent stomas were local recurrence [odd ratio (OR), 5.050; 95% confidence interval (CI), 1.867-13.659; p=0.001] and anastomotic-related complications (OR, 4.369; 95% CI, 1.631-11.701; p=0.001) (Table 2). The multivariate analysis revealed that sex, tumor-node-metastasis stage, and permanent stomas were independent prognostic factors for disease-free survival (Table 3).
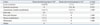
The characteristics of patients who needed a permanent stoma, including stoma recreation after reversal (stoma recreation group, n=14) and stoma non-reversal after diversion (stoma non-reversal group, n=14) are shown in Table 4. There were no differences between the two groups with respect to age, sex, body mass index, American Society of Anesthesiologists score, tumor location, stage, radiotherapy, anastomotic-related complications, systemic metastasis, or local recurrence. The reasons for requiring permanent stomas are compared between the two groups in Table 5. The main reason for permanent stomas in the stoma recreation group was local recurrence, whereas in the stoma non-reversal group, the most common reasons were anastomotic-related complications. However, this difference between the two groups was not significant. With respect to anastomotic-related complications between the two groups, the stoma recreation group was associated with late complications more often than early complications (early:late, 0:5) compared with the stoma non-reversal group (early:late, 2:3).
Survival data for the temporary and permanent stoma groups are presented in Fig. 2. Five-year disease-free survival and overall survival curves were significantly different between the two groups, respectively (both p<0.001).
Although it remains controversial whether diversion decreases the incidence of anastomotic leakage, its use is nevertheless widespread due to the possibility of mitigating the consequences of anastomotic complications. However, diverting stomas are associated with the potential risk of requiring a permanent stoma in two outcomes, namely, non-reversal of the temporary stoma and recreation of the stoma after reversal.
The overall rate of permanent stomas after sphincter-saving surgery of the rectum has been reported to be 3-24%.10,11,12,13,14 In addition, some studies have shown that the non-reversal rate of diverting loop ileostomy is 13.8-24.9%.9,15,16,17,18 In the present study, 12.1% of patients (28 of 231) had a permanent stoma, while 6.1% (14 of 231) had no reversals of diverting loop ileostomy. We also found that anastomotic-related complications and local recurrence were independent risk factors of permanent stomas in our study.
Local recurrence is a well-known risk factor of permanent stomas. Indeed, three recent studies reported that local recurrence is the main reason for needing a permanent stoma and thus is more frequent than anastomosis-related complications.11,16,19,20 In the present study, local recurrence was also the primary cause of the need for a permanent stoma and was followed by anastomotic related complications. Additionally, the primary cause was the same for stoma recreation cases after reversal, though not for stoma non-reversal after diversion. Lim, et al.20 reported similar results, indicating that local recurrence is the main reason for requiring stoma recreation after stoma closure. Specifically, they reported that 10 (55.6%) of 18 patients with permanent stomas due to local recurrence had received stoma recreation surgery.
Several recent studies have reported that anastomotic complications are predictive factors of permanent stomas. Junginger, et al.16 reported that the main reasons for needing a permanent stoma are anastomosis-related complications and local recurrence and that anastomosis-related complications are the most commonly cited reason for allowing non-functioning stomas to remain in place (20 of 33 patients). In the present study, anastomotic-related complications were the second most common reason for requiring a permanent stoma; however, they were the primary reason for stoma non-reversal after diversion (5 of 14). Seo, et al.19 reported similar data showing the primary causes of non-reversal of diverting stomas to be anastomotic-related complications, which were also the main reasons for early permanent stomas (<1 year after surgery).
Additional risk factors of permanent stomas include old age, radiation therapy, poor anal function, and distant metastasis. Interestingly, several previous studies have reported that old age is one of most significant risk factors for permanent stomas,10,15,21 while others have reported that it is not a risk factor.16,19,20 den Dulk, et al.15 suggested that fears of increased comorbidity in the elderly and patients' refusals to undergo additional surgeries may be responsible for the decreased frequency of stoma reversal in older patients. In addition, some studies have shown that radiation therapy may also be a risk factor for permanent stomas12,19 due to the association between radiotherapy and anastomotic-related complications.12 Conversely, Nelson, et al.11 reported that radiation therapy was found not to be a risk factor of permanent stomas. We concur with this finding after observing that radiation therapy was not a risk factor of permanent stoma in our study. Poor anal function and distant metastasis may also be risk factors for permanent stomas; however, these were not evaluated in this retrospective analysis. Specifically, we did not include these possible risk factors given that postoperative anal function was not evaluated as an objective measure in all patients at our institution and distant metastasis may have significantly influenced the high rate of permanent stomas due to disease progression.
The timing of stoma closure is highly variable. Some investigators reported an average period between primary surgery and stoma closure of 4-5.6 months.15,16,20 In the present study, the median time to stoma reversal was 7.5 months, which was a relatively longer interval to stoma reversal than what described in other reports. One of the possible reasons may have been a delay of stoma reversal after completion of postoperative adjuvant chemotherapy, which took place for 6 months.
The limitations of the current study include its retrospective and non-randomized design as well as the small number of patients in the permanent stoma group. In addition, the indications for diverting stomas were not standardized and instead depended on the surgeons' preferences, which may have resulted in selection bias. Lastly, as we did not use an objective measure, we also did not evaluate anal function, which may be an important risk factor for permanent stomas. Although our study is not substantial enough to make definite conclusions, the conclusions that we did come to are still valid.
In conclusion, patients with rectal cancer who have had low anterior resection with a temporary stoma and experience local recurrence or anastomotic-related complications may be at increased risk for a permanent stoma. Thus, it is necessary to be aware of the potential risks for the creation of a permanent stoma in these patients.
Figures and Tables
Fig. 2
Kaplan-Meier curves of 231 patients with temporary stomas. (A) Disease-free survival. (B) Overall survival.

Table 1
Risk Factors for a Permanent Stoma among 231 Temporary Stoma Patients
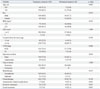
Table 2
Multivariate Analysis of Risk Factors for a Permanent Stoma

| OR (95% CI) | p value | |
|---|---|---|
| BMI | 0.693 (0.296-1.627) | 0.400 |
| Chemotherapy | 1.350 (0.517-3.524) | 0.539 |
| Anastomotic-related complications | 4.369 (1.631-11.701) | 0.003 |
| Local recurrence | 5.050 (1.867-13.659) | 0.001 |
Table 3
Univariate and Multivariate Analysis for Disease-Free Survival in 231 Patients with Temporary Stomas
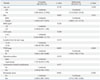
Table 4
Demographics of Permanent Stoma Patients
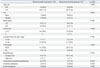
Table 5
Reasons for Requiring a Permanent Stoma
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number 2012R1A1A1004888).
References
1. Knight CD, Griffen FD. An improved technique for low anterior resection of the rectum using the EEA stapler. Surgery. 1980; 88:710–714.
3. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986; 1:1479–1482.

4. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–1740.

5. Matthiessen P, Hallböök O, Rutegård J, Sjödahl R. Population-based study of risk factors for postoperative death after anterior resection of the rectum. Br J Surg. 2006; 93:498–503.

6. Bokey EL, Chapuis PH, Hughes WJ, Koorey SG, Hinder JM, Edwards R. Morbidity, mortality and survival following resection for carcinoma of the rectum at Concord Hospital. Aust N Z J Surg. 1990; 60:253–259.

7. Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg. 2009; 96:462–472.

8. Pakkastie TE, Ovaska JT, Pekkala ES, Luukkonen PE, Järvinen HJ. A randomised study of colostomies in low colorectal anastomoses. Eur J Surg. 1997; 163:929–933.
9. Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007; 246:207–214.

10. Lindgren R, Hallböök O, Rutegård J, Sjödahl R, Matthiessen P. What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial. Dis Colon Rectum. 2011; 54:41–47.

11. Nelson RS, Boland E, Ewing BM, Blatchford GJ, Ternent C, Shashidharan M, et al. Permanent diversion rates after neoadjuvant therapy and coloanal anastomosis for rectal cancer. Am J Surg. 2009; 198:765–770.

12. Hassan I, Larson DW, Wolff BG, Cima RR, Chua HK, Hahnloser D, et al. Impact of pelvic radiotherapy on morbidity and durability of sphincter preservation after coloanal anastomosis for rectal cancers. Dis Colon Rectum. 2008; 51:32–37.

13. Maggiori L, Bretagnol F, Lefèvre JH, Ferron M, Vicaut E, Panis Y. Conservative management is associated with a decreased risk of definitive stoma after anastomotic leakage complicating sphincter-saving resection for rectal cancer. Colorectal Dis. 2011; 13:632–637.

14. Mala T, Nesbakken A. Morbidity related to the use of a protective stoma in anterior resection for rectal cancer. Colorectal Dis. 2008; 10:785–788.

15. den Dulk M, Smit M, Peeters KC, Kranenbarg EM, Rutten HJ, Wiggers T, et al. A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. Lancet Oncol. 2007; 8:297–303.

16. Junginger T, Gönner U, Trinh TT, Lollert A, Oberholzer K, Berres M. Permanent stoma after low anterior resection for rectal cancer. Dis Colon Rectum. 2010; 53:1632–1639.

17. David GG, Slavin JP, Willmott S, Corless DJ, Khan AU, Selvasekar CR. Loop ileostomy following anterior resection: is it really temporary. Colorectal Dis. 2010; 12:428–432.
18. Chun LJ, Haigh PI, Tam MS, Abbas MA. Defunctioning loop ileostomy for pelvic anastomoses: predictors of morbidity and nonclosure. Dis Colon Rectum. 2012; 55:167–174.
19. Seo SI, Yu CS, Kim GS, Lee JL, Yoon YS, Kim CW, et al. Characteristics and risk factors associated with permanent stomas after sphincter-saving resection for rectal cancer. World J Surg. 2013; 37:2490–2496.

20. Lim SW, Kim HJ, Kim CH, Huh JW, Kim YJ, Kim HR. Risk factors for permanent stoma after low anterior resection for rectal cancer. Langenbecks Arch Surg. 2013; 398:259–264.

21. Kairaluoma M, Rissanen H, Kultti V, Mecklin JP, Kellokumpu I. Outcome of temporary stomas. A prospective study of temporary intestinal stomas constructed between 1989 and 1996. Dig Surg. 2002; 19:45–51.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download