Abstract
Purpose
To compare the efficacy of the new drug calfactant with the commonly used drugs surfactant-TA and poractant alfa.
Materials and Methods
A total of 332 preterm infants at 24-31 weeks' gestation with respiratory distress syndrome (RDS) were enrolled and allocated to three groups according to the surfactant instilled; Group 1 (n=146, surfactant-TA), Group 2 (n=96, calfactant), and Group 3 (n=90, poractant alfa). The diagnosis of RDS and the decision to replace the pulmonary surfactant were left to the attending physician and based on patient severity determined by chest radiography and blood gas analysis. Data were collected and reviewed retrospectively using patient medical records.
Results
Demographic factors including gestational age, birth weight, Apgar score, clinical risk index for babies II score, and maternal status before delivery were not different between the study groups. Instances of surfactant redosing and pulmonary air leaks, as well as duration of mechanical ventilation, were also not different. Rates of patent ductus arteriosus, intraventricular hemorrhage (≥grade III), periventricular leukomalacia, high stage retinopathy of prematurity, necrotizing enterocolitis (≥stage II), and mortality were also similar, as was duration of hospital stay. Cases of pulmonary hemorrhage and moderate to severe bronchopulmonary dysplasia were increased in Group 3.
Exogenous surfactant replacement therapy has been the only effective treatment for preterm infants with respiratory distress syndrome (RDS) since 1990 and has markedly reduced pneumothorax and mortality.1,2 Preterm infants born before 32 weeks' gestation have structurally immature lungs at the saccular stage of development. Therefore, surface area and diffusion distance for gas exchange are not normal in these preterm infants. Furthermore, preterm infants with RDS have insufficient available surfactant pools, as well as immature surfactant composition and function. The combination of structural immaturity and an insufficient surfactant pool for gas exchange in preterm infants results in RDS.3 Surfactant of the mature lung is composed of surfactant-specific proteins and lipids, such as dipalmitoylphosphatidylcholine, surfactant protein (SP)-A, SP-B, SP-C, and SP-D, phosphatidylglycerol, and plasmalogen. Hydrophobic surfactant proteins, SP-B and SP-C play a significant role in the adsorption and spread of dipalmitoylphosphatidylcholine and in stabilizing alveoli.4
The first successful exogenous surfactant administration in newborn infants with RDS was reported by Fujiwara, et al.1 in 1980, who derived the surfactant from minced bovine lung. After the first report by Fujiwara, et al.,1 subsequent trials of surfactant replacement have been performed, and several animal-derived natural surfactants were made.5 Animal-derived surfactants are expensive and production is limited due to animal availability. Also, these surfactants contain foreign proteins that may be potentially immunogenic and infectious.6 Synthetic surfactants, which are free of animal proteins, may have advantages over animal-derived surfactants due to their lack of immune reactions, proinflammatory mediators causing bronchopulmonary dysplasia (BPD), and animal-borne infectious agents.6 They are also reproducible and have fewer production limitations than animal-derived surfactants. Nevertheless, a meta-analysis by Soll and Blanco7 in 2001 showed that protein-free old generation synthetic surfactants, such as colfosceril or pumactant, were associated with increased mortality and a greater risk of pneumothorax when compared to animal-derived surfactants. The inferiority of old generation synthetic surfactants is due to their absence of SP-B and SP-C, resulting in failure to lower surface tension. Thus, these protein-free old generation synthetic surfactants have dropped out of the market and are no longer used.
Three animal-derived surfactant preparations commonly used nationwide in Korea include surfactant-TA (Surfacten®, Mitsubishi-Tokyo Pharma Corporation, Osaka, Japan), calfactant (Infasurf®, ONY Inc., Amherst, NY, USA), and poractant alfa (Curosurf®, Chiesi Farmaceutici SpA, Parma, Italy). In Korea, Surfacten® came into the market in 1990, Curosurf® in 1996, and recently, Infasurf® in 2009. There are no published studies comparing efficacy and mortality in preterm infants treated with these three animal-derived surfactants in Korea. Randomized controlled trials using primary outcomes such as efficacy and mortality require a long period of time to complete along with a large sample size and considerable costs. To overcome these obstacles, we performed a retrospective study to evaluate whether differences could be found in efficacy, complications, and mortality among three pulmonary surfactants.
The protocol of this study was reviewed and approved by the Institutional Review Board of Busan Paik Hospital (13-010). Preterm infants of 24-31 weeks' gestation were enrolled; these subjects had been admitted to the neonatal intensive care units (NICU) of Busan Paik Hospital between January 2009 and December 2012 and diagnosed with RDS requiring pulmonary surfactant replacement therapy. Preterm infants who had a chromosomal abnormality or life-threatening major congenital malformation, such as cardiac anomaly or pulmonary hypoplasia, were excluded. These conditions acted as confounding factors by interfering with respiratory function and survival rate and were thus unsuitable for evaluating the efficacy of surfactants. The infants were allocated to three groups according to the type of surfactant instilled: Group 1, Surfacten®; Group 2, Infasurf®; Group 3, Curosurf®.
Clinical data were collected retrospectively from medical records. Surfactant was administered as rescue therapy until December 2010; thus, the diagnosis of RDS and the decision to replace pulmonary surfactant were left to the attending physician and were based on patient severity determined by chest radiography and blood gas analysis.8,9 From January 2011, surfactant was administered as prophylactic therapy in infants <30 weeks' gestation in the delivery room or operation room, according to notification No. 2010-135 of the Ministry of Health and Welfare on January 2011. We chose surfactants in alphabetical order (Curosurf®, Infasurf®, and then Surfacten®), and calfactant was used from 2010 in our NICU.
Surfactants were instilled into the trachea via an endotracheal tube using an orogastric tube. According to the prescribing information for each drug, Surfacten® (Mitsubishi-Tokyo Pharma Corporation, Osaka, Japan) was dissolved in 4 mL of normal saline and instilled at 4 mL/kg (120 mg/kg). Curosurf® (Chiesi Farmaceutici SpA, Parma, Italy) was administered at 2.5 mL/kg (200 mg/kg), and Infasurf® (ONY Inc., Amherst, NY, USA) was administered at 3 mL/kg (105 mg/kg).
Infant and maternal demographic factors included gestational age, birth weight, gender, Apgar score, small for gestational age (SGA), clinical risk index for babies (CRIB) II score, antenatal corticosteroids therapy, maternal pregnancy-induced hypertension, gestational diabetes mellitus, and histologically confirmed chorioamnionitis. Outcomes associated with RDS included a need for surfactant redosing, pulmonary air leak, pulmonary hemorrhage, mechanical ventilation (including non-invasive ventilation such as nasal continuous positive airway pressure), invasive ventilation (or intubation), postnatal steroids therapy, and BPD.
Outcomes associated with prematurity included patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), sepsis, duration of hospital stay, and mortality.
The definition of SGA was a birth weight less than the tenth percentile on the Lubchenco growth curve.10 Pulmonary air leak included pneumothorax, pneumomediastinum, or pulmonary interstitial emphysema. Pulmonary hemorrhage was diagnosed when bright red blood was spouted out of the endotracheal tube with typical chest radiographic findings and rapid deterioration of the patient. BPD was defined as an oxygen dependency at 36 weeks post-menstrual age with oxygen treatment for at least the first 28 days of life; this disorder was categorized by severity. Mild BPD was defined as breathing room air at 36 weeks post-menstrual age or discharge; moderate BPD was defined as a need for <30% O2 at 36 weeks post-menstrual age or discharge; and severe BPD was defined as a need for >30% O2 with or without positive pressure ventilation or continuous positive pressure at 36 weeks post-menstrual age or discharge.11 IVH and PVL were diagnosed by brain ultrasound and limited to high grade (≥grade III) IVH.12 ROP was limited to cases requiring laser therapy.13 NEC was defined using the modified Bell staging criteria and was limited to stage II and stage III.14 Sepsis was diagnosed upon clinical signs of systemic infection with a positive blood culture.15
For continuous variables with a normal distribution and homogeneous variance, the ANOVA test with Bonferroni correction was performed for comparison of multiple groups. For variables without a normal distribution or without homogeneous variance, such as gestational age, the Kruskal-Wallis test was performed. The chi-squared test was performed for nominal variables. Multivariate analysis with Bonferroni correction was performed to compare statistically significant variables. All data were analyzed using SAS Enterprise Guide 3.0 (SAS Institute, Cary, NC, USA). Data are given as mean±standard deviation, and p-values <0.05 were considered significant.
A total of 332 preterm infants were enrolled and allocated to three groups [Group 1 (Surfacten®): n=146; Group 2 (Infasurf®): n=96; Group 3 (Curosurf®): n=90].
There were no differences in gestational age, birth weight, gender, Apgar score at 1 and 5 minutes, SGA, CRIB II score, antenatal corticosteroids, antenatal antibiotics, antenatal magnesium sulfate therapy, maternal gestational diabetes mellitus, and chorioamnionitis between the groups. Maternal pregnancy-induced hypertension was higher in Group 3 than in Groups 1 and 2 (Table 1).
Gestational age was 28+1±2+1 weeks, 28+3±2+1 weeks, and 28+0±2+2 weeks, and birth weight was 1145±312 g, 1155±384 g, and 1088±372 g in Groups 1, 2, and 3, respectively.
The need for surfactant redosing was not different between the study groups [Group 1: 25 (17%); Group 2: 16 (17%); Group 3: 21 (23%); p=0.412]. Cases of pulmonary air leak [Group 1: 3 (2%); Group 2: 3 (3%); Group 3: 2 (2%); p=0.861], total duration of mechanical ventilation (Group 1: 15±16 days; Group 2: 16±18 days; Group 3: 17±19 days; p=0.785), duration of invasive ventilation (Group 1: 10±12 days; Group 2: 12±14 days; Group 3: 12±13 days; p=0.889), and cases of postnatal steroid therapy were also similar between the study groups. However, instances of pulmonary hemorrhage, moderate to severe BPD [Group 1: 8 (6%); Group 2: 5 (5%); Group 3: 12 (15%); p=0.041], and postnatal diuretic therapy in Group 3 were higher than Groups 1 and 2 (Table 2).
Rates of PDA and ligation of PDA, high grade IVH (≥grade III), PVL, high stage ROP requiring laser therapy, NEC (≥stage II), and mortality were similar between the study groups, as was duration of hospital stay. Sepsis was higher in Group 3 than in Groups 1 and 2 (Table 3).
In this study, we compared the clinical efficacy of calfactant (Infasurf®), which came into the market recently, with the commonly used surfactants in Korea, surfactant-TA (Surfacten®) and poractant alfa (Curosurf®) among preterm infants with RDS.
Surfactant-TA (Surfacten®) is derived from a minced bovine lung extract to which synthetic lipids are added to make it similar to natural lung surfactants. It has smaller amounts of phospholipids, SP-B, and plasmalogen than calfactant.2 Calfactant (Infasurf®) is a bovine lung lavage preparation that has higher amounts of phospholipids and SP-B than surfactant-TA.2 Poractant alfa (Curosurf®) is derived from a minced porcine lung extract that contains the highest amount of plasmalogen; some studies reported that it is associated with a decreased risk of BPD.16
Ramanathan17 reported that poractant alfa was associated with decreased mortality rates compared to beractant or calfactant, suggesting that the differences in mortality rates may be related to the composition of surfactants, such as higher amounts of phospholipids and plasmalogens and a smaller volume of poractant alfa than other animal-derived surfactants. A meta-analysis by Singh, et al.18 showed reduced mortality and redosing rates with poractant alfa at 200 mg/kg compared with beractant. However, the reduction was not significant for poractant alfa at 100 mg/kg compared with beractant. They suggested that the greater amounts of phospholipid, SP-B, and SP-C in 200 mg/kg of poractant alfa may have resulted in better outcome regardless of the source of the surfactants. A retrospective observational cohort study in the US compared poractant alfa with calfactant and beractant.19 They also concluded that poractant alfa at 200 mg/kg was associated with reduced mortality rates compared with calfactant at 105 mg/kg or beractant at 100 mg/kg. The same dose of poractant alfa and beractant at 100 mg/kg resulted in no difference in mortality rates, and mortality rates were also similar when comparing calfactant and beractant.
Trembath, et al.20 conducted a retrospective multicenter study in 2013. A total of 51282 infants admitted to 322 NICUs in the US received surfactant replacement with beractant, calfactant, or poractant alfa. There were no differences in outcomes such as air leak syndromes, BPD, NEC, IVH (grade III or IV), and mortality. The authors concluded that the differences in mortality and outcomes between surfactants, found in a large number of previous studies, do not demonstrate the true differences in the effectiveness of surfactants but are related to the outcome variations attributable to different institutions. The study by Trembath, et al. was a multicenter study with a large cohort of infants; however, it was a retrospective study rather than a randomized prospective study. Additionally, infants less than 37 weeks' gestation were included, the median gestational age was 30 weeks, and the median birth weight was 1435 g. In our study, preterm infants less than 32 weeks' gestation were included and had a mean gestational age of 28+1 weeks and a mean birth weight of 1130 g; therefore, they were both less mature and smaller than the infants studied by Trembath, et al. Thus, the results by Trembath, et al. cannot be equally applied to all preterm infants with RDS.
In our study, rates of maternal pregnancy-induced hypertension, pulmonary hemorrhage, and moderate to severe BPD were higher in Group 3, the poractant alfa group. As our study was not a randomized prospective study, the clinical data were collected retrospectively. Therefore, we could not avoid selection bias despite the lack of differences between demographic factors including gestational age, birth weight, Apgar score, CRIB II score, and maternal status before delivery. The CRIB score is a risk-adjustment instrument used widely in the NICU. To minimize the problems of treatment bias of the CRIB score, the CRIB II score was developed as an updated and simplified measurement in relation to mortality and major morbidity.21 In our study, CRIB II scores were similar between groups (Group 1: 8.1±3.7; Group 2: 7.8±3.9; Group 3: 8.5±4.0; p=0.367). According to the first National Institute of Child Health and Human Development Neonatal Research Network study in the US, most early deaths occurred at 22 and 23 weeks, and the mortality rate and morbidities such as IVH (≥grade III) markedly increased between 22 and 23 weeks [mortality rate: 94% at 22 weeks, 74% at 23 weeks, and 45% at 24 weeks; IVH (≥grade III): 38% at 22 weeks, 36% at 23 weeks, and 26% at 24 weeks].22 Therefore, we excluded infants at 22 and 23 weeks' gestation and only compared those at ≥24 weeks' gestation among the three study groups. If severe infants were enrolled more in Group 3 than in Groups 1 and 2, this would indicate inevitable selection bias as a shortcoming of this retrospective study, despite adjusting for confounding variables and finding similarities in gestational age, birth weight, Apgar scores, and CRIB II scores between groups. Thus, randomized prospective studies comparing the efficacies of animal-derived surfactants are required.
Protein-free old generation synthetic surfactants are not used due to the absence of SP-B and SP-C, as well as failure to lower surface tension, as previously mentioned in the Introduction. Lucinactant, a next-generation synthetic surfactant containing a protein analog of SP-B in animal-derived surfactants, has been developed; this has been found to reduce RDS, compared with colfosceril or other old generation synthetic surfactants, although there was no difference when compared with beractant.23 Lucinactant also reduced BPD more than colfosceril and decreased RDS-related mortality more than both colfosceril and beractant. Lucinactant was also found to be more effective than colfosceril in the prevention of RDS in the "Lucinactant Trials."23 A 1-year follow-up study of the Lucinactant Trials concluded that lucinactant was "just as good, if not superior, to animal-derived surfactants in the prevention of RDS and may be a viable alternative to animal-derived products."24 Thus far, animal-derived surfactants seem to be better than lucinactant for the treatment of RDS and for the prevention and decrease of complications related to RDS, such as pulmonary air leak, BPD, and mortality. Further comparative prospective studies between animal-derived surfactants and next-generation synthetic surfactants such as lucinactant are required.
RDS causes significant mortality in preterm infants; thus, exogenous surfactant replacement has been the only effective treatment for RDS and has markedly decreased mortality rates of preterm infants with RDS. Therefore, surfactant replacement therapy in RDS is very important, and the most effective surfactant must be chosen. While a large number of studies and meta-analyses comparing the effectiveness of surfactants have been performed, they have yielded conflicting results.
In this study, calfactant (Infasurf®), which entered the market recently in Korea, was found to be equally as effective as surfactant-TA (Surfacten®) and poractant alfa (Curosurf®). Further randomized prospective studies comparing these surfactants are required. This is the first study comparing the efficacy of surfactant-TA, calfactant, and poractant alfa in a large number of preterm infants in Korea.
Figures and Tables
Table 1
Infant and Maternal Demographic Factors
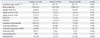
Table 2
Outcomes Associated with RDS
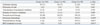
Table 3
Outcomes Associated with Prematurity

References
1. Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980; 1:55–59.

3. Engle WA. American Academy of Pediatrics Committee on Fetus and Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008; 121:419–432.

4. Logan JW, Moya FR. Animal-derived surfactants for the treatment and prevention of neonatal respiratory distress syndrome: summary of clinical trials. Ther Clin Risk Manag. 2009; 5:251–260.
6. Moya F. Synthetic surfactants: where are we? Evidence from randomized, controlled clinical trials. J Perinatol. 2009; 29:Suppl 2. S23–S28.

7. Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2001; CD000144.

8. Jobe A. Respiratory distress syndrome--new therapeutic approaches to a complex pathophysiology. Adv Pediatr. 1983; 30:93–130.
9. Jobe AH. 50 Years ago in The Journal of Pediatrics: Respiratory distress syndrome of newborn infants: I. Diagnosis and incidence Miller H. J Pediatr 1962;61:2-8. Respiratory Distress Syndrome of Newborn Infants: II. Clinical Study of Pathogenesis Miller H. J Pediatr 1962;61:9-16. J Pediatr. 2012; 161:93.
10. Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from Liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics. 1963; 32:793–800.

12. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–534.

13. An international classification of retinopathy of prematurity. Pediatrics. 1984; 74:127–133.
14. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986; 33:179–201.

15. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004; 292:2357–2365.

16. Rüdiger M, von Baehr A, Haupt R, Wauer RR, Rüstow B. Preterm infants with high polyunsaturated fatty acid and plasmalogen content in tracheal aspirates develop bronchopulmonary dysplasia less often. Crit Care Med. 2000; 28:1572–1577.

17. Ramanathan R. Animal-derived surfactants: where are we? The evidence from randomized, controlled clinical trials. J Perinatol. 2009; 29:Suppl 2. S38–S43.

18. Singh N, Hawley KL, Viswanathan K. Efficacy of porcine versus bovine surfactants for preterm newborns with respiratory distress syndrome: systematic review and meta-analysis. Pediatrics. 2011; 128:e1588–e1595.

19. Ramanathan R, Bhatia JJ, Sekar K, Ernst FR. Mortality in preterm infants with respiratory distress syndrome treated with poractant alfa, calfactant or beractant: a retrospective study. J Perinatol. 2013; 33:119–125.

20. Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013; 163:955–960.

21. Parry G, Tucker J, Tarnow-Mordi W. UK Neonatal Staffing Study Collaborative Group. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003; 361:1789–1791.

22. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010; 126:443–456.

23. Moya FR, Gadzinowski J, Bancalari E, Salinas V, Kopelman B, Bancalari A, et al. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics. 2005; 115:1018–1029.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download