Abstract
Purpose
In this study, we investigated the stroke mechanism and the factors associated with ischemic stroke in patients with nonvalvular atrial fibrillation (NVAF) who were on optimal oral anticoagulation with warfarin.
Materials and Methods
This was a multicenter case-control study. The cases were consecutive patients with NVAF who developed cerebral infarction or transient ischemic attack (TIA) while on warfarin therapy with an international normalized ratio (INR) ≥2 between January 2007 and December 2011. The controls were patients with NVAF without ischemic stroke who were on warfarin therapy for more than 1 year with a mean INR ≥2 during the same time period. We also determined etiologic mechanisms of stroke in cases.
Results
Among 3569 consecutive patients with cerebral infarction or TIA who had NVAF, 55 (1.5%) patients had INR ≥2 at admission. The most common stroke mechanism was cardioembolism (76.0%). Multivariate analysis demonstrated that smoking and history of previous ischemic stroke were independently associated with cases. High CHADS2 score (≥3) or CHA2DS2-VASc score (≥5), in particular, with previous ischemic stroke along with ≥1 point of other components of CHADS2 score or ≥3 points of other components of CHA2DS2-VASc score was a significant predictor for development of ischemic stroke.
Nonvalvular atrial fibrillation (NVAF) is the most common cause of cardioembolic stroke.1,2 Warfarin (vitamin K antagonist) treatment with optimum intensity has been a standard treatment to prevent ischemic stroke or systemic embolism in patients with NVAF.3,4,5
However, despite having a therapeutic intensity of oral anticoagulation (OAC) therapy, some patients still suffer from ischemic stroke.6 Some patients may have a hypercoagulable state, and subsequently intractable to appropriate OAC therapy.7,8 Furthermore, noncardioembolic stroke such as atherothrombotic stroke or lacunar infarction can occur in patients with NVAF,9,10 and these types of ischemic stroke may not be successfully prevented with OAC. Until now, however, there has been limited information regarding the clinical determinants for development of ischemic stroke and the stroke mechanism in patients with NVAF who were on warfarin therapy in real clinical setting.
In this study, we investigated which factors are associated with the development of ischemic stroke in patients with NVAF who are on optimal OAC therapy. We also determined the etiologic mechanisms of stroke in them.
This study was a multicenter, case-control study with prospective case ascertainment and retrospective data collection between January 2007 and December 2011. Cases and controls were collected from total 14 hospitals in South Korea. This study was approved by the Institutional Review Board at each participating hospital and the requirement for informed consent was waived.
Consecutive patients with cerebral infarction or transient ischemic attack (TIA) who were admitted to study hospitals during study period were investigated for eligibility for this study. All patients underwent brain CT/MRI, or both. During admission, demographic data, medical history, vascular risk factors, and clinical manifestations were collected for each patient. Among patients with cerebral infarction or TIA, a patient was eligible as the cases if they had NVAF with prior warfarin therapy and their international normalized ratio (INR) was ≥2 at presentation of ischemic stroke or TIA.
Controls were selected from patients who were treated in the department of neurology or cardiology at each study hospital during the same time period. We reviewed the medical records and results of laboratory tests. Patients were included as controls when they had NVAF and received warfarin for more than 1 year with a mean INR ≥2. Controls should not have a cerebral infarction or TIA for one year while they had been on warfarin therapy with their mean INR ≥2. Controls were matched 1:1 to cases on age and gender.
We collected data for demographics, vascular risk factors, underlying cardiovascular diseases, and concurrent medications in cases and controls. Atrial fibrillation (AF) was categorized as paroxysmal or persistent/permanent AF. The CHADS2 score or CHA2DS2-VASc scores were calculated for all patients. The index cerebral infarction or TIA was not considered for calculating the CHADS2 score or CHA2DS2-VASc score. Concomitant potential cardiac sources of embolism was defined as having at least one of left atrial/atrial appendage thrombus, sick sinus syndrome, recent myocardial infarction (<4 weeks), left ventricular thrombus, dilated cardiomyopathy, and akinetic left ventricular segment, along with NVAF. Patients were regarded as smokers if they smoked within the 3 months period prior to admission. INR data were also collected; the INR at admission for cases and the mean INR during the 1 year prior to enrollment for controls were used in the analysis. We also calculated time in therapeutic range by the method of Rosendaal, et al.11 among the patients enrolled as controls (mean number of checking INR=6). However, we could not calculate time in therapeutic range in cases because all patients included in the case group had not followed up before index stroke at each hospital.
We collected data of location of ischemic lesions and the initial stroke severity assessed by the National Institute of Health Stroke Scale in cases. In patients with cerebral infarction, we determined the stroke mechanism, which was based on the Trial of Org 10172 in Acute Stroke Treatment classification.12 Two neurologists (YDK and HSN) independently reviewed brain imaging including the diffusion weighted images and angiographic studies. If the classification was conflicting, consensus was reached through the discussion together with the third investigator (KYL).
Statistical analysis was performed using the Windows SPSS package (version 18.0, SPSS Inc., Chicago, IL, USA) and the SAS package (version 9.1.3, SAS Inc., Cary, NC, USA). Fisher's exact chi-square test was used for categorical variables and independent t-test or Kruskal-Wallis test for continuous variables, as appropriate. In the investigation of determinants for cerebral infarction or TIA on therapeutic warfarin treatment between the case and control group, categorical variables were compared with McNemar test or exact McNemar test and continuous variables with paired t-tests or Wilcoxon signed-rank test, as appropriate. To find independent factors that were associated with development of cerebral infarction or TIA while on optimal warfarin therapy, a conditional logistic regression analysis was performed using variables with p<0.05 on univariate analysis. Statistical significance was set at p<0.05.
During the study period, a total of 25415 consecutive patients with cerebral infarction or TIA were admitted to study hospitals. Among them, after exclusion of patients without NVAF, those without previous OAC therapy, and those whose INR was <2, 55 patients (50 patients with cerebral infarction and 5 patients with TIA) were included in this study as cases (Fig. 1). The location of cerebral infarction was most common in the middle cerebral artery territory (35/50, 70.0%), followed by multiple arterial territories (9/50, 18.0%), brainstem (5/50, 10.0%), and posterior cerebral artery territory (1/50, 2.0%). Among 50 cases with cerebral infarction, their median National Institutes of Health Stroke Scale (NIHSS) score at admission was 3 [interquartile range (IQR) 1-11].
Among 55 patients included in the case group, we could determine the stroke mechanism in 50 patients because 5 patients had no ischemic lesion on diffusion weighted imaging. Among cases with cerebral infarction (n=50), diffusion weighted MRI was performed in 48 patients (96.0%) and angiographic studies were done in 49 patients (98.0%). Of them, 38 patients (76.0%) were classified as cardioembolic stroke and 12 patients (24.0%) as stroke of undetermined etiology due to multiple causes [cardioembolism (CE)+large artery atherosclerosis in 9 patients (18.0%) and CE+lacunar infarction in 3 patients (6.0%)]. In comparison of baseline characteristics between groups according to stroke mechanism, CE was significantly associated with the presence of congestive heart failure, while there were no differences in the age, gender, risk factors, and previous medication before index stroke (Table 1). Neurologic deficits at admission was more severe in CE (median NIHSS score at admission 4, IQR 2-15) compared to those in CE+large artery atherosclerosis (median 1, IQR 0-3) or CE+lacunar infarction (median 1, IQR 0.5-1.5) (p=0.022).
The comparison between cases and controls is shown in Table 2. Cases were more likely to be smokers, and more frequently had a history of previous ischemic stroke or TIA. The CHADS2 score or CHA2DS2-VASc score was higher in cases than in controls (p<0.001 at each comparison). Other baseline characteristics were comparable between two groups. Median time in therapeutic range in control group was 64.2% (IQR 49.2-81.8%).
Multivariate analysis showed that smoking and history of previous ischemic stroke were independently associated with the occurrence of cerebral infarction or TIA (Table 3). Cases with CHADS2 score ≥3 or CHA2DS2-VASc score ≥5 had significantly higher odds ratio of having cerebral infarction or TIA than patients with a CHADS2 score or CHA2DS2-VASc score of 0-1 (Table 3).
The previous ischemic stroke or TIA, which contributed two points to the CHADS2/CHA2DS2-VASc score, was a strong determinant for the cases while CHADS2 score ≥3 was related with the cases. Therefore, we divided the study population into four groups according to the presence of previous ischemic stroke or TIA as follows; 1) CHADS2 score ≤2 but without previous ischemic strokes or TIA (n= 42); 2) CHADS2 score ≥3 but without previous ischemic strokes or TIA (n=29); 3) CHADS2 score 2 and previous ischemic strokes or TIA (n=2); 4) CHADS2 score ≥3 and previous ischemic strokes or TIA (n=37). Likewise, the study population was also divided to four groups according to CHA2DS2-VASc score and the previous ischemic strokes or TIA; 1) CHA2DS2-VASc score ≤4 but without previous ischemic strokes or TIA (n=49); 2) CHA2DS2-VASc score ≥5 but without previous ischemic strokes or TIA (n=22); 3) CHA2DS2-VASc score ≤4 and previous ischemic strokes or TIA (n=10); 4) CHA2DS2-VASc score ≥5 and previous ischemic strokes or TIA (n=29). After adjusting smoking, the CHADS2 score ≥3 with previous ischemic strokes or TIA were significantly associated with cases, while the CHADS2 score ≥3 without previous ischemic strokes or TIA was not. Likewise, higher CHA2DS2-VASc scores with previous ischemic strokes or TIA were more significantly related with cases than higher CHA2DS2-VASc scores without previous ischemic strokes or TIA were (Table 4).
Despite optimal warfarin treatment, cerebral infarction or TIA can occur in patients with NVAF. Among patients with NVAF who received warfarin in large contemporary randomized controlled trials, the rate of stroke or non-central nervous system embolism was 1.2% to 2.3% per year.13 In our study, of 683 ischemic stroke or TIA patients with known NVAF and OAC therapy, 8.1% (n=55) had INR ≥2.0, which is similar to that [7.9% (44/556)] in Fukoka Stroke Registry.14
Occurrence of stroke in patients with NVAF who are on warfarin with therapeutic intensity may be ascribed to other causes such as atherosclerosis and lacunar infarction. Cerebral artery atherosclerosis co-exists in 20-50% of patients with NVAF and may develop stroke.9,15 Likewise, lacunar infarction may develop in patients with NVAF.16 However, our results demonstrated that most ischemic strokes (76%) during OAC developed due to CE.
Considering positive association between risk stratification schemes and prothrombotic conditions, it was expected that higher CHADS2 scores or CHA2DS2-VASc scores were related with the risk of ischemic stroke or TIA during warfarin treatment. However, even though the CHADS2 score or CHA2DS2-VASc score was same, the risk of stroke was substantially different according to the presence of a history of previous stroke or TIA. Although the scores on the risk stratification were similarly high (CHADS2 ≥3 or CHA2DS2-VASc ≥5), the risk for ischemic stroke was 3.81 times (CHADS2 ≥3) and 3 times (CHA2DS2-VASc ≥5) higher in patients with a history of previous stroke or TIA than in those without history of previous stroke or TIA.
Our findings suggest that warfarin treatment with INR 2.0-3.0 may be insufficient to effectively prevent stroke in patients with higher CHADS2 score (≥3) or CHA2DS2-VASc score (≥5) with a previous stroke or TIA. In these patients, more potent antithrombotic measures can be considered. Current guidelines mention that it may be reasonable to raise the intensity of warfarin to a maximum target INR of 3.0-3.5, rather than add an antiplatelet agent in patients with AF who sustain ischemic stroke or systemic embolism during warfarin treatment with INR 2.0-3.0.17 However, meta-analysis showed that new oral anticoagulants (NOACs) seemed to be associated with a significant reduction (relative risk reduction 14%) in rates of stroke or systemic embolism in patients with previous stroke or TIA, compared to warfarin treatment.18 Because stroke rate during OAC was reported to be higher in Asians than non-Asians and NOAC seems to be more effective than warfarin in Asians than non-Asians,19,20 NOAC may have some advantage of treating this specific group. Recently, local therapy of left atrial appendage closure with Watchman device showed some potential as alternatives of OAC.21 New trials targeting this particular group of patients would be necessary to determine which treatment strategy is beneficial in them.
In this study, smoking was independently associated with the risk of cerebral infarction or TIA. However, previous studies have not consistently shown positive relationship between smoking and the risks of stroke in patients with NVAF.22,23,24 Intracardiac thrombus formation in NVAF is affected by endocardial damage, inflammation, and platelet activation as well as blood stasis in the left atrium.25 Smoking can inhibit secretion of endogenous tissue plasminogen activator, increase the plasminogen activator inhibitor-1 activity, induce systemic inflammation, activate platelets, and damage endothelial cells, which may subsequently lead to a hypercoagulable state.26,27 Smoking is a modifiable risk factor.28 Our results suggest that cessation of smoking is essential to further reduce stroke risks in patients with NVAF who are treated with OAC.
There are some limitations of our study. Although this study was a multicenter case-control study, the data were collected retrospectively. Second, we could not investigate the effect of age and gender due to the study design that matched age and gender between cases and controls. Third, because this study was a retrospective cross-sectional design, our results cannot prove a causal relationship between high CHADS2/CHA2DS2-VASc scores and a previous ischemic stroke or smoking and stroke risk. Moreover, the control group was selected if patients had no stroke before 1 year during OAC, and the sample size of our study was small and there is a potential for selection bias, hence the results cannot be generalized.
For calculation of CHADS2 scores and CHA2DS2-VASc scores, 2 points are given to NVAF patients with a history of stroke TIA, and oral anticoagulation use is recommended in them. However, we showed that patients with a stroke TIA are at very high risk of recurrent stroke despite warfarin treatment as is recommended, particularly if their CHADS2 scores are ≥3 or CHA2DS2-VASc scores are ≥5. Our findings suggest that some other measures to reduce the risk of stroke sould be determined in the future in those specific groups of patients.
Figures and Tables
Fig. 1
Patient selection. TIA, transient ischemic attack; NVAF, nonvalvular atrial fibrillation; INR, international normalized ratio.

Table 1
Baseline Characteristics of Cerebral Infarction Cases According to Stroke Mechanisms
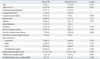
Table 2
Comparison of Baseline Characteristics between Cases and Controls
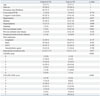
Table 3
Conditional Logistic Regression Analysis for Ischemic Strokes and TIA

Table 4
Conditional Logistic Regression Analysis According to the Presence of Previous Strokes or TIA

ACKNOWLEDGEMENTS
This work was supported by a grant of the Korea Healthcare Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI10C2020).
References
1. Cardiogenic brain embolism. Cerebral Embolism Task Force. Arch Neurol. 1986; 43:71–84.
2. Han SW, Nam HS, Kim SH, Lee JY, Lee KY, Heo JH. Frequency and significance of cardiac sources of embolism in the TOAST classification. Cerebrovasc Dis. 2007; 24:463–468.

3. Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA 3rd, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011; 57:223–242.
4. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011; 42:227–276.

5. European Stroke Organisation (ESO) Executive Committee. ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008; 25:457–507.
6. Paciaroni M, Agnelli G, Ageno W, Caso V, Corea F, Lanari A, et al. Risk factors for cerebral ischemic events in patients with atrial fibrillation on warfarin for stroke prevention. Atherosclerosis. 2010; 212:564–566.

7. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864–2870.

8. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010; 137:263–272.

9. Kim YD, Cha MJ, Kim J, Lee DH, Lee HS, Nam CM, et al. Increases in cerebral atherosclerosis according to CHADS2 scores in patients with stroke with nonvalvular atrial fibrillation. Stroke. 2011; 42:930–934.

10. Melkas S, Putaala J, Oksala NK, Pohjasvaara T, Oksala A, Kaste M, et al. Small-vessel disease relates to poor poststroke survival in a 12-year follow-up. Neurology. 2011; 76:734–739.

11. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69:236–239.

12. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.

13. Agarwal S, Hachamovitch R, Menon V. Current trial-associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta-analysis. Arch Intern Med. 2012; 172:623–631.

14. Nakamura A, Ago T, Kamouchi M, Hata J, Matsuo R, Kuroda J, et al. Intensity of anticoagulation and clinical outcomes in acute cardioembolic stroke: the Fukuoka Stroke Registry. Stroke. 2013; 44:3239–3242.

15. Kanter MC, Tegeler CH, Pearce LA, Weinberger J, Feinberg WM, Anderson DC, et al. Carotid stenosis in patients with atrial fibrillation. Prevalence, risk factors, and relationship to stroke in the Stroke Prevention in Atrial Fibrillation Study. Arch Intern Med. 1994; 154:1372–1377.

16. Evans A, Perez I, Yu G, Kalra L. Should stroke subtype influence anticoagulation decisions to prevent recurrence in stroke patients with atrial fibrillation? Stroke. 2001; 32:2828–2832.

17. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011; 123:e269–e367.
18. Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012; 43:3298–3304.

19. Albertsen IE, Rasmussen LH, Overvad TF, Graungaard T, Larsen TB, Lip GY. Risk of stroke or systemic embolism in atrial fibrillation patients treated with warfarin: a systematic review and meta-analysis. Stroke. 2013; 44:1329–1336.

20. Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014; 111:789–797.

21. Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013; 127:720–729.

22. Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010; 41:2731–2738.

23. Novello P, Ajmar G, Bianchini D, Bo GP, Cammarata S, Firpo MP, et al. Ischemic stroke and atrial fibrillation. A clinical study. Ital J Neurol Sci. 1993; 14:571–576.

24. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994; 154:1449–1457.
25. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009; 373:155–166.

26. Johnson CM, Mureebe L, Silver D. Hypercoagulable states: a review. Vasc Endovascular Surg. 2005; 39:123–133.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download