Abstract
Purpose
Magnetic resonance imaging (MRI) has been used to screen and follow-up spinal dural arteriovenous fistulae (SDAVF). The purpose of this study was to evaluate the association between MRI findings and neurologic function in SDAVF. This study also investigated clinical features and treatment results of SDAVF.
Materials and Methods
A total of 15 consecutive patients who underwent embolization or surgery for SDAVF were included. We treated seven (60%) patients with embolization and six (40%) with surgery. We analysed clinical features, MRI findings, treatment results, and neurologic function. Neurologic function was measured by the Aminoff-Logue disability scale (ALS).
Results
Patients with longer levels of intramedullary high signal intensity in preoperative T2-weighted images (T2WI) exhibited worse pre- and postoperative ALS scores (r=0.557, p=0.031; r=0.530, p=0.042, Pearson correlation). Preoperative ALS score was significantly correlated with postoperative ALS score (r=0.908, p=0.000, Pearson correlation). The number of levels showing intramedullary high signal intensity in T2WI decreased significantly postoperatively (5.2±3.1 vs. 1.0±1.4, p=0.001, Wilcoxon ranked test).
Arteriovenous malformation is a rare condition in the spine and affects approximately 10% of the brain.1 Among spinal arteriovenous malformations, spinal dural arteriovenous fistulae (SDAVF) are the most common and account for 70% of cases.2 Treatment is often necessary, as abnormal communication between arteries and veins may result in ischemia or infarction in the spinal cord;2 however, its rarity limits proper treatment planning and precise outcome prediction.3 Although angiography is the most precise diagnostic tool for SDAVF, it may cause procedural complications, such as puncture site haematoma, intra- and extra-spinal vessel injury, and spinal cord infarction. Furthermore, angiography uses a large amount of radiation and usually requires a short hospital admission. Therefore, magnetic resonance imaging (MRI) has been used to screen and follow up SDAVF. Its advantages include good visualization of the pathological anatomy, no risk of harmful radiation, and a non-invasive nature.4 There are numerous studies describing the MRI findings of SDAVF, including oedema, infarction, flow voids, and perimedullary venous engorgement.3,4,5,6 To the best of our knowledge, however, there is a lack of literature regarding the correlation between MRI findings and neurologic function. The purpose of this study was to evaluate the association between MRI findings and the functional status of SDAVF. This study also investigated the clinical features and surgical results of SDAVF.
A total of 15 consecutive patients underwent embolization or surgery for SDAVF in our institution from 2002 to 2007. Institutional Review Board approval for the study was obtained. All medical records and imaging studies were reviewed and analysed. Two independent physicians reviewed MRI and angiography findings. The last follow-up was completed via a telephone encounter. The mean age was 48 years, ranging 19 to 66 years. There were 13 men and 2 women. The mean follow-up period was 27 months, ranging from 18 to 36 months.
MRI (Signa HD 1.5T, GE, Waukesha, WI, USA) was taken preoperatively and one year after treatment in all patients with the following parameters: TR/TE=400-600/10-20 for T1-weighted images, with or without gadolinium (Gd) enhancement, and TR/TE=3000-3400/100-120 for T2-weighted images (T2WI). Two independent radiologists reviewed the MRI findings. If a value was different between the two observers, the more severe one was selected. The extent of high T2WI was calculated as the involved vertebral levels on sagittal images. We graded the severity of flow voids of the perimedullary vessels on sagittal images as Grade 0, none; Grade 1, indefinite; Grade 2, definite; and Grade 3, severe.
We treated seven (60%) patients with embolization only and six (40%) patients with surgery. We attempted embolization first in all subjects because of its non-invasiveness, the opportunity for instant treatment following diagnostic angiography, and the shorter hospitalization time.7 Angiography was performed under local anaesthesia. A mixture of N-butyl 2-cyanoacrylate and lipiodol was injected to the point of the fistula in cases where an arterialized feeder was confirmed. In one case, coil embolization was added (Fig. 1). When the endovascular approach to the fistula point was not possible or the radicular artery was directly supplying the anterior spinal artery, open surgery was performed under general anaesthesia. Following laminotomy, the dura was opened and the fistula was identified by tracing a dorsal engorged vein into a nerve root sleeve. Next, we cauterized the fistula point with a bipolar coagulator. The treatment result was determined as complete or partial obliteration by postoperative angiography. The surgical outcome was classified by the Odom criteria as follows: excellent=complete resolution; good=partial improvement; fair=no change; poor=progression.
Functional status was measured by the Aminoff-Logue disability scale (ALS). The ALS consists of three categories: gait, bladder, and bowel function. Gait was graded as follows: G0=normal; G1=leg weakness, abnormal gait or stan-ce, or no restricted activity; G2=restricted activity but no support required; G3=one stick required for walking; G4=two sticks or crutches, or frame required for walking; G5=con-fined to wheelchair. Bladder function was graded as follows: M0=normal; M1=infrequent hesitancy or urgency, altered sensation, but continent; M2=occasional urinary incontinence or retention; M3=total incontinence or persistent retention. Bowel function was graded as follows: B0=normal; B1=moderate constipation; B2=severe constipation or occasional incontinence; B3=total incontinence.
Clinical findings are summarized in Table 1. Motor weakness was the most common presenting symptom (n=12, 80%), followed by neurogenic bladder (n=1, 7%) and sensory hypaesthesia (n=1, 7%). One case (7%) was incidentally diagnosed without any symptoms during a routine health examination. The mean symptom duration was 15 months, ranging from 0 to 96 months. There was no statistically significant difference between the symptom duration and the pre- and postoperative ALS scores (p=0.108, p=0.226, Pearson correlation). All except three cases showed a slowly progressive course. Three cases showed rapid progression with a symptom duration less than 1 month. Among them, two cases showed spinal haemorrhage (Case 9 and Case 12), while the other presented as spinal ischemia (Case 1).
SDAVFs occurred most commonly at the thoracic spine (n=10), followed by the lumbosacral (n=3), and thoracolumbar spine (n=2). The T2-weighted MRI revealed high signal intensity in the spinal cord in 14 of 15 cases. Flow void was observed in 14 of 15 cases in the dorsal surface of the spinal cord. When flow void was indefinite in the T2-weighted MRI, enhanced T1-weighted MRI with Gd was a good alternative to reveal arterialized perimedullary veins. Patients with longer levels of intramedullary high signal intensity in preoperative T2WI had worse preoperative and postoperative ALS scores (r=0.557, p=0.031; r=0.530, p=0.042, Pearson correlation) (Fig. 2).
Complete obliteration was achieved in 13 cases (87%). The average ALS score improved significantly following treatments (7.0±3.3 vs. 5.7±3.4, p=0.007, Wilcoxon ranked test) (Fig. 3, Table 2). By the Odom scale, nine patients (60%) showed a good result. There were five cases (33%) of a fair result and one case (7%) of a poor result. Among the five cases of a fair result, four cases had poor ALS scores preoperatively and one case had a long symptom duration (96 months). The preoperative ALS score was significantly correlated with the postoperative ALS score (r=0.908, p=0.000, Pearson correlation). There was one case of temporary cord ischemia in which spinal cord infarction developed due to glue migration. We heparinised the patient for 2 days. The patient eventually recovered to the preoperative state.
The number of levels showing intramedullary high signal intensity in T2WI decreased significantly postoperatively (5.2±3.1 vs. 1.0±1.4, p=0.001, Wilcoxon ranked test); however, the level of intramedullary high signal intensity in postoperative T2WI had no correlation with postoperative ALS score (r=0.220, p=0.431, Pearson correlation). Even though SDAVF was completely obliterated, the perimedullary veins were still engorged in postoperative MRI (n=6, 40%). This may have stemmed from the irreversible structural change of the perimedullary vein.
This study aimed to elucidate the association between the MRI findings and functional status of SDAVF. The clinical features and surgical outcomes were also reviewed. All of our patients underwent pre- and post-operative angiography and MRI. All SDAVF were initially screened by MRI as in a previous study.5 Complete obliteration was achieved in 87% of cases, including embolization and surgery. The series had favourable results, with 60% of cases showing symptom relief and a 6.7% rate of temporary complications. This result was similar to the meta-analysis by Steinmetz, et al.8
This study confirmed that longer levels of intramedullary high signal intensity in preoperative T2WI are associated with worse pre- and postoperative functional status. The high signal intensity significantly decreased the following treatment. This result is consistent with the previous study of Horikoshi, et al.5 They reported that preoperative MRI may predict treatment outcome, despite the fact that MRI may not visualize shunts or flow voids in SDAVF. High signal intensity in T2WI is thought to be indicative of spinal cord oedema.2,5,9,10,11 It rarely presents hemorrhage, but is more commonly identified with myelopathy. SDAVF can cause venous congestion by direct drainage from the radiculomeningeal artery to the perimedullary venous plexus.12,13,14 Consequently, ischemia or infarction can occur, resulting in spinal cord oedema.12,13 If the high signal intensity involves more levels, more serious spinal cord oedema may exist. Consistent with this idea, we can expect a worse functional outcome if the high signal intensity remains; however, we could not find an association between high signal intensity in postoperative T2WI and functional outcome. Similar to our results, Horikoshi, et al.5 also reported that the extent of the high-intensity area did not correlate with postoperative neurological deficits; however, this may have resulted from their small sample size. It has been reported that postoperative MRI may represent functional status and give evidence of recurrence.9,15,16 Further study is essential to elucidate this hypothesis.
This study showed that poor functional outcomes are associated with longer symptom duration. This is consistent with previous studies. Kohno, et al.9 revealed that the functional outcome was better in shorter symptom duration groups. Additionally, postoperative functional status was closely associated with preoperative functional status. This has been uniformly observed in various spinal cord lesions.3,5,17,18,19,20 Among preoperative function measures, Wakao, et al.3 reported that only gait was correlated with postoperative functional status; however, our study showed that all functions, including gait, bladder, and bowel, are correlated with postoperative functional status.
This study has limitations mainly stemming from its small sample size. Life quality assessment such as SF-36 was not included. Treatments were heterogeneous, including three types of treatment. Nevertheless, this study is valuable in terms of elucidating the association between intramedullary high T2WI and functional outcome.
The number of involved levels of high signal intensity in preoperative T2WI is useful for predicting the pre- and postoperative functional status of SDAVF.
Figures and Tables
Fig. 1
Illustrative case (Case 5). A 49-year-old male presented with progressive motor weakness for 18 months. (A) Preoperative T2-weighted MRI showed spinal cord oedema and venous engorgement. (B) Preoperative angiography. The fistula is located on the dorsal surface of the spinal cord, with a large arterialized vein emanating from the nerve root sleeve. (C) Postoperative MRI shows complete resolution of spinal cord oedema and venous engorgement. (D) Postoperative angiography. The proximal draining vein and fistula are embolized. Postoperative DSA image (anteroposterior view) demonstrating obliteration of the fistula.

Fig. 2
The number of involved levels showing high signal intensity in T2WI vs. pre- and postoperative Aminoff-Logue disability scale (ALS) scores. T2WI, T2-weighted images.

Table 1
Summary of Clinical and Treatment Profiles
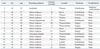
Table 2
Aminoff-Logue Disability Scale (ALS) Score and Magnetic Resonance Imaging (MRI) Findings

References
1. da Costa L, Dehdashti AR, terBrugge KG. Spinal cord vascular shunts: spinal cord vascular malformations and dural arteriovenous fistulas. Neurosurg Focus. 2009; 26:E6.

2. Koch C, Kucinski T, Eckert B, Röther J, Zeumer H. [Spinal dural arteriovenous fistula: clinical and radiological findings in 54 patients]. Rofo. 2003; 175:1071–1078.
3. Wakao N, Imagama S, Ito Z, Ando K, Hirano K, Tauchi R, et al. Clinical outcome of treatments for spinal dural arteriovenous fistulas: results of multivariate analysis and review of the literature. Spine (Phila Pa 1976). 2012; 37:482–488.
4. Dehdashti AR, Da Costa LB, terBrugge KG, Willinsky RA, Tymianski M, Wallace MC. Overview of the current role of endovascular and surgical treatment in spinal dural arteriovenous fistulas. Neurosurg Focus. 2009; 26:E8.

5. Horikoshi T, Hida K, Iwasaki Y, Abe H, Akino M. Chronological changes in MRI findings of spinal dural arteriovenous fistula. Surg Neurol. 2000; 53:243–249.

6. Luetmer PH, Lane JI, Gilbertson JR, Bernstein MA, Huston J 3rd, Atkinson JL. Preangiographic evaluation of spinal dural arteriovenous fistulas with elliptic centric contrast-enhanced MR Angiography and effect on radiation dose and volume of iodinated contrast material. AJNR Am J Neuroradiol. 2005; 26:711–718.
7. Narvid J, Hetts SW, Larsen D, Neuhaus J, Singh TP, McSwain H, et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008; 62:159–166.
8. Steinmetz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ, et al. Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta-analysis. Neurosurgery. 2004; 55:77–87.

9. Kohno M, Takahashi H, H S, Sasaki T, Ishijima B. Functional prognosis after treatment of spinal dural arteriovenous fistulas. J Clin Neurosci. 1998; 5 Suppl:12–15.

10. Terwey B, Becker H, Thron AK, Vahldiek G. Gadolinium-DTPA enhanced MR imaging of spinal dural arteriovenous fistulas. J Comput Assist Tomogr. 1989; 13:30–37.

11. Willinsky RA, terBrugge K, Montanera W, Mikulis D, Wallace MC. Posttreatment MR findings in spinal dural arteriovenous malformations. AJNR Am J Neuroradiol. 1995; 16:2063–2071.
12. Aminoff MJ, Barnard RO, Logue V. The pathophysiology of spinal vascular malformations. J Neurol Sci. 1974; 23:255–263.

13. Hassler W, Thron A, Grote EH. Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study. J Neurosurg. 1989; 70:360–370.
14. Kendall BE, Logue V. Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology. 1977; 13:181–189.

15. Isu T, Iwasaki Y, Akino M, Koyanagi I, Abe H. Magnetic resonance imaging in cases of spinal dural arteriovenous malformation. Neurosurgery. 1989; 24:919–923.

16. Gokhale S, Khan SA, McDonagh DL, Britz G. Comparison of surgical and endovascular approach in management of spinal dural arteriovenous fistulas: a single center experience of 27 patients. Surg Neurol Int. 2014; 5:7.

17. Fisher CG, Noonan VK, Smith DE, Wing PC, Dvorak MF, Kwon BK. Motor recovery, functional status, and health-related quality of life in patients with complete spinal cord injuries. Spine (Phila Pa 1976). 2005; 30:2200–2207.

18. Ropper AE, Gross BA, Du R. Surgical treatment of Type I spinal dural arteriovenous fistulas. Neurosurg Focus. 2012; 32:E3.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download