Abstract
Purpose
The purpose of the present study was to identify more useful parameters for predicting behaviors of multifocal papillary thyroid carcinoma (PTC).
Materials and Methods
We investigated dominant tumor diameter (TD), total tumor diameter (TTD), and total surface area (TSA) in tumors from 588 patients and evaluated their usefulness as parameters for assessment of tumor behaviors in multifocal PTCs.
Results
In 588 PTCs, tumor multifocality was found in 179 PTCs (30.4%). Multifocal tumors were significantly associated with extrathyroidal extension, lymph node metastasis, and higher tumor stage grouping (p<0.001, p<0.001, and p<0.001, respectively). The rates of nodal metastasis increased with greater TSA and TTD in PTCs. Multifocal papillary thyroid microcarcinomas (mPMCs) with TSA >3.14 cm2 had higher rates of nodal metastasis than mPMCs with TSA ≤3.14 cm2 (p=0.038); however, there was no significant difference between mPMCs with TTD >1.0 cm and with TTD ≤1.0 cm (p=0.325). In addition, nodal metastasis was more frequent in mPMCs with TSA >3.14 cm2 than in unifocal papillary thyroid microcarcinomas (uPMCs) (TD ≤1.0 cm) (p=0.002), but not overt unifocal PTCs (TD >1.0 cm) (p=0.244).
Papillary thyroid carcinoma (PTC) is the most common malignant tumor of the endocrine organs. Papillary thyroid microcarcinoma (PMC) of the thyroid is defined as a PTC of 1 cm or less in greatest diameter, according to the World Health Organization classification system.1 PMCs are more common than overt PTCs (larger than 1 cm) and have a more favorable prognosis. Nevertheless, for PMCs, unfavorable factors affecting aggressive behavior and prognosis are unclear.
Multifocal tumors may represent either multiple independent primary tumors or intraglandular dissemination from a primary tumor,2 and tumor multifocality has been found in 20 to 40% of PMCs.3,4,5,6,7,8 Lymph node metastasis has been observed at higher rates in multifocal PMCs (mPMCs) than in unifocal PMCs (uPMCs).8,9 Based on the American Thyroid Association management guidelines,10 hemithyroidectomy or subtotal thyroidectomy is generally recommended as adequate treatment for uPMCs; however, in multifocal PMCs, surgical treatment and postoperative management are controversial.
In the present study, we investigated dominant tumor diameter (TD), total tumor diameter (TTD), and total surface area (TSA) in multifocal PTCs. We evaluated the clinicopathological significance of tumor multifocality and the role of TSA as a new parameter for assessing tumor behavior in PTC.
Five hundred and eighty-eight consecutively resected classical variants of PTC at the Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine (Seoul, Korea) from January 1, 2010 to December 31, 2011 were analyzed. The correlation between tumor multifocality and clinicopathological characteristics was evaluated by reviewing medical charts, pathological records, and glass slides, following the 7th edition of American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) classification.11 The pathological features were reviewed by two pathologists. The patients' mean age was 45.5±11.4 years (range 19-81 years); mean tumor size was 0.98±0.61 cm; and patients had undergone either total thyroidectomy (n=431) or lobectomy (n=157) with lymph node dissection. Central node dissection was performed in all patients, and modified radical neck dissection was selectively performed in patients confirmed as having metastasis in the lateral neck lymph nodes via preoperative fine-needle aspiration biopsy or frozen section (n=32). In terms of pathologic TNM stage grouping, there were 381 cases in stage I, one in stage II, 195 in stage III, and 11 in stage IV. All 298 patients under 45 years old in the present study were classified as stage I. This protocol was reviewed and approved by the Institutional Review Board of Kangbuk Samsung Hospital (approval No. KBC13145).
In our institution, sections were cut to a thickness of 0.2 cm for pathologic examination. All tumors detected during gross examination and representative sections with normal-looking parenchyma from each lobe were histologically examined.
The multifocal tumors may represent either multiple independent primary tumors or intraglandular dissemination from a primary tumor.12 In this study, regardless of origin, multifocal tumors were defined as tumors with a distance between each tumor greater than 0.5 cm.
In the present study, the dominant tumor was defined as the largest single tumor for multifocal PTC or the primary for unifocal PTC. In multifocal tumors, TTD and TSA were defined as the total combined diameter and the surface area of each tumor, respectively.
The shape of PTCs was mostly ovoid to round, with dimensions taller than they were wide. We estimated tumor surface area by the following formula:13
where a, b, and c are the perpendicular diameter of each tumor focus with a>b>c. In the present study, three-dimensional diameters were measured on gross and microscopic examination as shown in Fig. 1. PMC (TD ≤1.0 cm) entails a more favorable prognosis than overt PTC (TD >1.0 cm). In addition, previous studies have reported that PMC with TD >0.5 cm show more aggressive behaviors than PMC with TD ≤0.5 cm.14,15,16,17 Thus, in consideration of tumor behavior, we divided subgroups into PTCs with TD ≤0.5 cm, PTCs with 0.5 cm<TD≤1.0 cm, and PTCs with TD >1.0 cm, respectively. We assigned TD 0.5 cm to TSA 0.785 cm2 and TD 1.0 cm to TSA 3.14 cm2.
All statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). Correlation between tumor multifocality and clinicopathological parameters was determined by either the χ2 test or the Fisher exact test (two-sided). In addition, using TD, TTD, and TSA, the correlation between tumor multifocality and lymph node metastasis was investigated. Multivariate logistic regression analysis was performed to identify the most influential variables associated with lymph node metastasis. The results are reported as p-values and odds ratios (ORs) with 95% confidence intervals (CIs). All results with p<0.05 were considered statistically significant.
First, we investigated the clinicopathological significances of tumor multifocality in 588 classical PTCs. Tumor multifocality was noted in 179 of 588 PTCs (30.4%), in which overt PTCs showed a higher rate of tumor multifocality than PMCs (p=0.015). Tumor multifocality was significantly correlated with extrathyroidal extension (p<0.001), lymph node metastasis (p<0.001), and higher tumor stage (p<0.001) but not with age (p=0.055) or gender (p=0.901). There were differences between multifocal and unifocal PTCs in TSA (p<0.001) and TTD (p<0.001); however, there was no difference in TD (p=0.483) (Table 1). In multivariate analysis, multifocality was an independent predictor of lymph node metastasis (p<0.001, OR=2.227, 95% CI 1.554-3.192).
All PTCs were classified based on TSA and TTD, which represented PTCs with TD ≤0.5 cm, PTCs with 0.5 cm<TD≤1.0 cm and PTCs with TD >1.0 cm, respectively. TSA and TTD groups were subdivided as TSA ≤0.785 cm2, 0.785 cm2<TS≤3.14 cm2, and TSA >3.14 cm2, as well as TTD ≤0.5 cm, 0.5 cm<TTD≤1.0 cm, and TTD >1.0 cm, respectively. Lymph node metastasis was found in 36.4%, 42.3%, and 61.6% of each TSA group, respectively. Based on TTD groups, the nodal metastasis rates were 19.4%, 43.7%, and 64.9%, respectively. Nodal metastasis was found in a higher rate in the larger TSA and TTD groups than in the smaller groups (p<0.001 and p<0.001, respectively) (Table 2). In multivariate analysis, TSA and TTD were independent predictors of lymph node metastasis (p<0.001, OR=1.157, 95% CI 1.089-1.230 and p<0.001, OR=3.185, 95% CI 1.851-5.481, respectively).
Next, we evaluated the usefulness of TSA and TTD in distinguishing aggressive mPMCs from favorable mPMCs. The groups of TSA and TTD were subdivided based on criteria of overt PTCs (TD >1.0 cm), which represented TSA >3.14 cm2 and TTD >1.0 cm, respectively. Tumors with TSA >3.14 cm2 comprised 13 of 104 mPMCs (12.5%) and those with TTD >1.0 cm consisted of 47 of 104 mPMCs (45.2%). Lymph node metastasis was observed in 42 (46.2%) of 91 mPMCs with TSA ≤3.14 cm2 and 10 (76.9%) of 13 mPMCs with TSA >3.14 cm2. Multifocal PMCs with TSA >3.14 cm2 had higher rates of nodal metastasis than mPMCs with TSA ≤3.14 cm2 (p=0.038); however, there was no significant difference between mPMCs with TTD >1.0 cm and those with TTD ≤1.0 cm (p=0.325) (Table 3).
As shown in Table 4, uPMCs exhibited lymph node metastasis in 95 of 280 cases (33.9%). Nodal metastasis was found in higher rates in mPMCs with TSA ≤3.14 cm2 (p=0.036), as well as mPMCs with TSA >3.14 cm2 (p=0.002), than in uPMCs; however, there was no difference between mPMCs with TSA >3.14 cm2 and unifocal overt PTCs (p=0.370).
Tumor multifocality of PTCs has been shown to be correlated with aggressive behavior, such as extrathyroidal extension and lymph node metastasis; nevertheless, the management guidelines of mPMCs remain controversial. The present study is the first, to the best of our knowledge, to show that assessment using TSA could be useful in differentiating between aggressive and favorable mPMCs and in deciding postoperative management.
In our study, tumor multifocality was observed in 30.4% of PTCs and was significantly correlated with extrathyroidal extension, lymph node metastasis, and tumor stage grouping (Table 1). Although Katoh, et al.18 reported that multifocal tumors were found in 78.1% of all PTCs, tumor multifocality recently has been reported in only 20-40% of cases.3,4,5,6,7,8 Other studies have shown that tumor multifocality is significantly associated with lymph node metastasis, which is consistent with our results.19,20,21 The correlation between tumor multifocality and extrathyroidal extension is controversial.19,20,21 Lin, et al.20 and Mazeh, et al.22 reported that tumor multifocality is significantly correlated with tumor recurrence. In mPMCs, a significant correlation between tumor multifocality and lymph node metastasis has also been reported.8 According to previous reports and our results, tumor multifocality may be a high risk factor. However, tumor multifocality is not included in any staging system, including AGES (Age, Grade, Extent, Size), AMES (Age, Distant metastasis, Extent, Size), MACIS (Distant metastasis, Age, Completeness of resection, Local invasion, Size), GAMES (Grade, Age, Distant metastasis, Extent, Size), or AJCC TNM classification.11,23,24 Despite the significant correlation between tumor multifocality and aggressive behavior, little is known about the reason for its exclusion as a risk factor.
The prognosis for PMC is favorable, even in the presence of regional lymph node metastases and local invasion.25 The rates of locoregional and regional lymph node recurrence have been reported to be between 2% and 5.7% in PMCs.26,27,28 According to the American Thyroid Association management guidelines,10 lobectomy and/or central lymph node dissection is considered an adequate treatment for uPMCs without other higher risk features. Total thyroidectomy with lymph node dissection is recommended for overt PTCs with multifocality,10,22 however, optimal treatment guidelines for multifocal PMCs remain controversial. A subset of PMCs with aggressive behavior requires surgical treatment and postoperative management similar to overt PTCs.29,30 Thus, it is important to differentiate multifocal PMCs requiring more aggressive surgical treatment and postoperative management. In addition, more detailed assessment of different surgical treatment and postoperative management options for multifocal tumors are needed.
In the present study, tumor multifocality was observed at a higher rate in overt PTCs than it was in PMCs. Based on the 7th edition of the AJCC TNM classification systems,11 primary tumor (T) stage is to be evaluated via tumor size and extrathyroidal extension; however, in multifocal PTCs, tumor size is evaluated by the largest tumor size, regardless of tumor number. The evaluation of extrathyroidal extension is performed for all tumors, including the largest tumor and other smaller tumors. Although PTCs show higher rates of tumor multifocality than other tumors,14 little is known about the effects of tumor multifocality on aggressive behavior. Whether the second tumor of multifocal PTCs affects aggressive behavior remains a subject for further investigation.
The assessment parameters for surgical treatment and postoperative management of multifocal PMCs are not fully understood. In the assessment for tumor multifocality of PTCs, new trials have attempted to assess the use of total tumor diameter or tumor number. Zhao, et al.8 introduced TTD as an assessment tool; however, these results were not included in the comparison of lymph node metastasis between mPMCs with TTD >1 cm and with TTD <1 cm. Our results revealed no significant difference in lymph node metastasis rates between mPMCs with TTD >1 cm and with TTD ≤1 cm (Table 3). In addition, the shape of PTCs was mainly ovoid to round with dimensions taller than they were wide.31 Therefore, tumor diameter cannot accurately represent true tumor bulk, as tumor volume or total surface area can. Tumor surfaces are proportional to the second and third power of the diameter. By adding the diameters of multifocal tumors, TTD could overestimate total surface area. These results suggest that TTD cannot distinguish aggressive mPMCs from more favorable mPMCs and uPMCs. Lin, et al.20 reported that tumor number was significantly associated with tumor recurrence. The detection of multifocal tumors is dependent on surgical extent, degree of inspection, and microscopic examination;32 however, it is difficult to microscopically examine the entire thyroid gland, and tumor multifocality may be missed. In addition, if bilateral lobes are not examined through total thyroidectomy, tumors in the contralateral lobe may be missed.32 These seem to be unavoidable limitations for thorough detection of multifocal tumors. Accordingly, prediction of lymph node metastasis or tumor recurrence via tumor number has limitations.
In the present study, we evaluated the usefulness of a new parameter, TSA, in predicting nodal metastasis of PTCs. The criteria for TSA analysis were 0.785 cm2 and 3.14 cm2, which are equivalent to TD 0.5 cm and TD 1.0 cm, respectively. Regardless of tumor multifocality, the larger TSA group showed significantly higher rates of nodal metastasis than the smaller TSA group (Table 2). Although overt PTCs showed higher rates of lymph node metastasis than PMCs (p<0.001), there was no significant difference in nodal metastasis between mPMCs with TSA >3.14 cm2 and overt PTCs (p=0.465) (data not shown). In addition, as shown in Table 4, mPMCs with TSA >3.14 cm2 showed higher rates of lymph node metastasis than mPMCs with TSA ≤3.14 cm2 and uPMCs. Andea, et al.13,33 previously reported that the assessment using TSA in multifocal breast carcinomas and TSA rather than TTD was useful for the prediction of lymph node metastasis. According to our results, PTCs with TSA >3.14 cm2 showed higher rates of extrathyroidal extension than PTCs with TSA ≤3.14 cm2 (p<0.001). However, there was no significant difference in extrathyroidal extension between mPMCs with TSA >3.14 cm2 and mPMCs with TSA ≤3.14 cm2 (p=0.194). In the present study, three-dimensional diameters were measured on gross and microscopic examination. If a tumor has marked infiltrative border, the estimated surface area may be smaller than the real surface area, which would warrant consideration in practice. Notwithstanding, according to our results and previous reports, TSA rather than TTD could be useful for differentiating between higher and lower risk groups of nodal metastasis in multifocal tumors.
In conclusion, the present study showed that multifocal PMCs can be evaluated via TSA, and patients with mPMCs with TSA >3.14 cm2 may be managed in a different manner than patients with uPMCs or mPMCs with TSA ≤3.14 cm2. As a new parameter, TSA could be a useful parameter for deciding on preoperative, surgical, and postoperative management of mPTCs.
Figures and Tables
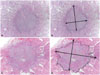 | Fig. 1Examples of measurements of tumor diameter. (A and B) Well-demarcated papillary thyroid carcinoma. (C and D) Papillary thyroid carcinoma with infiltrative border (×20). |
Table 2
Correlation between Total Surface Area and Total Tumor Diameter and Lymph Node Metastasis in Papillary Thyroid Carcinomas

References
1. DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization Classification of Tumours. Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press;2004. p. 57–66.
2. Shattuck TM, Westra WH, Ladenson PW, Arnold A. Independent clonal origins of distinct tumor foci in multifocal papillary thyroid carcinoma. N Engl J Med. 2005; 352:2406–2412.

3. Siassakos D, Gourgiotis S, Moustafellos P, Dimopoulos N, Hadjiyannakis E. Thyroid microcarcinoma during thyroidectomy. Singapore Med J. 2008; 49:23–25.
4. Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid. 2009; 19:707–716.

5. Ogilvie JB, Patel KN, Heller KS. Impact of the 2009 American Thyroid Association guidelines on the choice of operation for well-differentiated thyroid microcarcinomas. Surgery. 2010; 148:1222–1226.

6. Ciuffreda L, De Martino D, Bonfitto N, Scaramuzzi R. [Our experience on surgical treatment of papillary thyroid microcarcinoma]. G Chir. 2011; 32:41–44.
7. Connor MP, Wells D, Schmalbach CE. Variables predictive of bilateral occult papillary microcarcinoma following total thyroidectomy. Otolaryngol Head Neck Surg. 2011; 144:210–215.

8. Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X, et al. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol. 2013; 20:746–752.

9. Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg. 2009; 96:253–257.

10. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.

11. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag;2009. p. 87–100.
12. Pyo JS, Kang G, Kim DH, Park C, Kim JH, Sohn JH. The prognostic relevance of psammoma bodies and ultrasonographic intratumoral calcifications in papillary thyroid carcinoma. World J Surg. 2013; 37:2330–2335.

13. Andea AA, Bouwman D, Wallis T, Visscher DW. Correlation of tumor volume and surface area with lymph node status in patients with multifocal/multicentric breast carcinoma. Cancer. 2004; 100:20–27.

14. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003; 98:31–40.

15. Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005; 103:2269–2273.

16. Pelizzo MR, Boschin IM, Toniato A, Piotto A, Bernante P, Pagetta C, et al. Papillary thyroid microcarcinoma (PTMC): prognostic factors, management and outcome in 403 patients. Eur J Surg Oncol. 2006; 32:1144–1148.

17. Ghossein R, Ganly I, Biagini A, Robenshtok E, Rivera M, Tuttle RM. Prognostic factors in papillary microcarcinoma with emphasis on histologic subtyping: a clinicopathologic study of 148 cases. Thyroid. 2014; 24:245–253.

18. Katoh R, Sasaki J, Kurihara H, Suzuki K, Iida Y, Kawaoi A. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma. A clinicopathologic study of 105 consecutive patients. Cancer. 1992; 70:1585–1590.

19. Park SY, Park YJ, Lee YJ, Lee HS, Choi SH, Choe G, et al. Analysis of differential BRAF(V600E) mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer. 2006; 107:1831–1838.

20. Lin JD, Chao TC, Hsueh C, Kuo SF. High recurrent rate of multicentric papillary thyroid carcinoma. Ann Surg Oncol. 2009; 16:2609–2616.

21. Kim HJ, Kim NK, Choi JH, Kim SW, Jin SM, Suh S, et al. Radioactive iodine ablation does not prevent recurrences in patients with papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2013; 78:614–620.

22. Mazeh H, Samet Y, Hochstein D, Mizrahi I, Ariel I, Eid A, et al. Multifocality in well-differentiated thyroid carcinomas calls for total thyroidectomy. Am J Surg. 2011; 201:770–775.

23. Rodríguez-Cuevas S, Labastida-Almendaro S, Cortés-Arroyo H, López-Garza J, Barroso-Bravo S. Multifactorial analysis of survival and recurrences in differentiated thyroid cancer. Comparative evaluation of usefulness of AGES, MACIS, and risk group scores in Mexican population. J Exp Clin Cancer Res. 2002; 21:79–86.
24. Voutilainen PE, Siironen P, Franssila KO, Sivula A, Haapiainen RK, Haglund CH. AMES, MACIS and TNM prognostic classifications in papillary thyroid carcinoma. Anticancer Res. 2003; 23:4283–4288.
25. Appetecchia M, Scarcello G, Pucci E, Procaccini A. Outcome after treatment of papillary thyroid microcarcinoma. J Exp Clin Cancer Res. 2002; 21:159–164.
26. Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008; 144:980–987.

27. Besic N, Pilko G, Petric R, Hocevar M, Zgajnar J. Papillary thyroid microcarcinoma: prognostic factors and treatment. J Surg Oncol. 2008; 97:221–225.

28. Pelizzo MR, Boschin IM, Toniato A, Pagetta C, Piotto A, Bernante P, et al. Natural history, diagnosis, treatment and outcome of papillary thyroid microcarcinoma (PTMC): a mono-institutional 12-year experience. Nucl Med Commun. 2004; 25:547–552.

29. Lee J, Rhee Y, Lee S, Ahn CW, Cha BS, Kim KR, et al. Frequent, aggressive behaviors of thyroid microcarcinomas in Korean patients. Endocr J. 2006; 53:627–632.

30. Page C, Biet A, Boute P, Cuvelier P, Strunski V. 'Aggressive papillary' thyroid microcarcinoma. Eur Arch Otorhinolaryngol. 2009; 266:1959–1963.

31. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008; 247:762–770.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


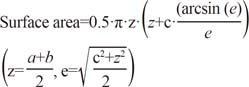


 XML Download
XML Download