Abstract
Purpose
Over the last 30 years, Serratia marcescens (S. marcescens) has emerged as an important pathogen, and a common cause of nosocomial infections. The aim of this study was to identify risk factors associated with mortality in patients with S. marcescens bacteremia.
Materials and Methods
We performed a retrospective cohort study of 98 patients who had one or more blood cultures positive for S. marcescens between January 2006 and December 2012 in a tertiary care hospital in Seoul, South Korea. Multiple risk factors were compared with association with 28-day all-cause mortality.
Results
The 28-day mortality was 22.4% (22/98 episodes). In a univariate analysis, the onset of bacteremia during the intensive care unit stay (p=0.020), serum albumin level (p=0.011), serum C-reactive protein level (p=0.041), presence of indwelling urinary catheter (p=0.023), and Sequential Oran Failure Assessment (SOFA) score at the onset of bacteremia (p<0.001) were significantly different between patients in the fatal and non-fatal groups. In a multivariate analysis, lower serum albumin level and an elevated SOFA score were independently associated with 28-day mortality [adjusted odds ratio (OR) 0.206, 95% confidential interval (CI) 0.044-0.960, p=0.040, and adjusted OR 1.474, 95% CI 1.200-1.810, p<0.001, respectively].
Serratia marcescens (S. marcescens) is a Gram-negative Enterobacteriaceae species, initially considered non-pathogenic due to its low virulence in healthy populations.1 Over the last 30 years, however, this species has emerged as an important pathogen, and a common cause of nosocomial infections.2 S. marcescens has been shown to cause a wide range of infectious diseases, including urinary, respiratory, and biliary tract infections, peritonitis, wound infections, and intravenous catheter-related infections, which can also lead to life-threatening bacteremia.1,2 Risk factors associated with these infections include prolonged immunosuppressive therapy, previous antimicrobial agents, indwelling catheterization, and underlying diseases such as chronic pulmonary disease and diabetes mellitus.3
Recent epidemiologic analyses have shown an increase in the rate of antimicrobial resistance among S. marcescens isolates.4,5,6 Furthermore, multidrug-resistant (MDR) strains of S. marcescens have been associated with serious outcomes.7,8,9 The overall mortality rate of S. marcescens bacteremia remains high, ranging from 25-58%.2,9,10 However, despite this high mortality rate, the risk factors associated with mortality in S. marcescens bacteremia have not been well established since 2008 regardless of MDR strains.10,11,12,13
Therefore, we aimed to identify the risk factors associated with mortality in patients with S. marcescens bacteremia during the last 6 years.
A retrospective cohort study was conducted to investigate risk factors associated with mortality in S. marcescens bacteremia at Severance Hospital, a 2000-bed, tertiary-care teaching hospital, Seoul, South Korea. Inclusion criteria for this study included patients with 18 years of age or older, identified as having one or more blood cultures positive for S. marcescens between January 2006 and December 2012. For subjects reporting more than one episode of S. marcescens bacteremia, only the first episode was accepted. Demographic and clinical variables were evaluated using microbiological laboratory records and clinical data gained from electronic medical records; these included age, gender, length of hospital stay, underlying diseases, predisposing conditions, portal of entry, appropriateness of antimicrobial agents, appropriateness of definitive therapy, results of antimicrobial susceptibility testing, laboratory data at the time of bacteremia onset, Sequential Organ Failure Assessment score (SOFA),14 and 28-day all-cause mortality. This study was approved by our Institutional Review Board. Informed consent was exempt from our local ethics committee because this study was concerned to cause minimal harm on persons.
Significant S. marcescens bacteremia was defined as S. marcescens isolates cultured from one or more blood samples obtained from a patient, combined with clinical symptoms compatible with systemic inflammatory response syndrome.15 Hospital-acquired bacteremia was defined as a positive blood culture taken from a patient no sooner than 48 h after hospital admission, whereas healthcare-associated bacteremia was defined as a positive blood culture taken from a patient receiving home and/or ambulatory intravenous therapy, hemodialysis, wound care, chemotherapy, or nursing care, or who had attended a hospital clinic within the last 30 days; patients hospitalized in an acute care hospital for ≥2 days within the last 90 days; or those living in a nursing home or long-term care facility.16 The primary site of infection was presumed to be the source of bacteremia if S. marcescens was identified from any culture specimens at the time of bacteremia onset; if S. marcescens was not identified from any culture other than the blood, the source was presumed to be primary bacteremia. Polymicrobial bacteremia was defined as bacteremia where more than one organism were isolated from the same blood culture specimen. Septic shock was defined as sepsis-induced organ hypo-perfusion, combined with either a systolic blood pressure <90 mm Hg or <40 mm Hg less than baseline, or a mean arterial pressure <65 mm Hg after a fluid resuscitation, eventually leading to require the vasopressor use.17 Underlying chronic diseases included hemato-oncological disease, chronic renal disease, chronic liver disease, chronic lung disease, cardiovascular disease, and cerebrovascular disease, as defined by the International Classification of Disease, 10th Revision.18 Prior use of antimicrobial agent was defined as receipt for at least 48 h within 1 month prior to the bacteremic episode. Appropriateness of initial empirical antimicrobial agents was defined as the use of at least one in vitro susceptible antimicrobial agent within 24 h of positive blood culture before the susceptibility was known.19 Definitive therapy was defined as antibiotic therapy given properly according to the results of final blood culture.20 Hypoalbuminemia was defined as a serum albumin of less than 3.0 g/dL at the time of bacteremia.21,22 Twenty-eight-day all-cause mortality was investigated to confirm the primary outcome.
Clinical isolates were evaluated using either conventional techniques or the ATB 32 GN system (bioMérieux, Marcy l' Etoile, France). Antimicrobial susceptibility testing was performed by microbiology laboratory staff using the disk-diffusion method or a VITEK-2 N131 card (bioMérieux, Hazelwood, MO, USA). Results were interpreted using the guidelines set forth by the Clinical and Laboratory Standards Institute.23
Student's t-test was used to compare continuous variables; and categorical variables were analyzed using either a χ2 or Fisher's exact test as appropriate. Nonparametric variables were analyzed using the Mann-Whitney U test. Univariate and multivariate analyses to evaluate independent risk factors for all-cause mortality in S. marcescens bacteremia were performed through the logistic regression models. Statistical analyses were performed using the SPSS software, version 20 (SPSS Inc., Chicago, IL, USA). p-values <0.05 were considered to indicate statistical significance; all values reported are for two-tailed analyses.
A total of 98 episodes of S. marcescens bacteremia were identified between January 2006 and December 2012. The annual distribution of S. marcescens bacteremia is shown in Fig. 1. To confirm the outbreak, the trend was investigated and stratified by ward and period, but there were no outbreaks.
Table 1 shows the demographics, clinical characteristics, and underlying conditions of all patients with S. marcescens bacteremia. Sixty patients (61.2%) were male, and the ages of patients ranged from 26 to 92 years (median age, 63.5 years). Of 98 bacteremic episodes, hospital-acquired bacteremia accounted for 73.5% of the episodes (72 patients), whereas healthcare-associated bacteremia accounted for 18.4% of the episodes (18 patients). The remaining 8 episodes (8.1%) were defined as community-acquired bacteremia. The most common underlying condition was malignancy (46.9%), which included conditions such as solid organ and hematologic malignancy; and diabetes mellitus (30.6%) were also common. The most frequent portal of entry was the lower respiratory tract (50%). Other portals of entry included the urinary tract (20.4%), abdomen (11.2%), skin and soft tissue (10.2%), and intra-venous catheter (5.1%). Half of the patients had central venous catheters (CVCs), including chemo-ports, and urinary catheters. 56.1% of the patients showed bacteremia during the intensive care unit (ICU) stay. Among them, the fatal group showed a greater incidence of bacteremia during the ICU stay (77.3%) than the non-fatal group (50%) (p=0.020). 46.9% of the episodes (46 patients) showed the 3rd cephalosporin resistance, but there was no statistically significant difference between the fatal and non-fatal group (p=0.744).
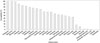
The antimicrobial susceptibilities of S. marcescens clinical isolates are characterized in Fig. 2. The majority of isolates were susceptible to ertapenem (100%), meropenem (99%), imipenem (93.4%), cefepime (87.8%), isepacin (81.8%), and ceftazidime (76.5%). A few isolates exhibited susceptibility to amoxicillin/clavulanic acid (1.1%), ampicillin (2.1%), and ampicillin/sulbactam (1.7%).
The 28-day all-cause mortality was 22.4% (22/98). Univariate analysis revealed significant differences in the number of important clinical covariates including the onset of bacteremia during the ICU stay (p=0.020), serum albumin level (p=0.011), serum C-reactive protein level (p=0.041), presence of indwelling urinary catheter (p=0.023), and SOFA score at the onset of bacteremia (p<0.001) between patients in the fatal and non-fatal groups (Table 1). In a multivariate analysis, lower serum albumin level [adjusted odds ratio (OR) 0.206, 95% confidential interval (CI) 0.044-0.960, p=0.040], and elevated SOFA score (adjusted OR 1.474, 95% CI 1.200-1.810, p<0.001) were all found to be independent risk factors for mortality in patients with S. marcescens bacteremia (Table 2).
A number of recent reports have shown that S. marcescens bacteremia may arise from both community-acquired as well as healthcare-associated exposures.10,12,13,24,25 Furthermore, an increase in the number of multidrug-resistant S. marcescens strains has been reported worldwide.26,27,28 As these factors can substantially influence the outcome of S. marcescens bacteremia, we sought to identify the risk factors associated with S. marcescens-related mortality during recent 6 years. In our study, the 28-day all-cause mortality rate was 22.4%, similar to that in a previous report from South Korea.12
In previous studies, a wide array of independent risk factors have been found to be associated with mortality in patients with S. marcescens bacteremia, including old age (>65 years), pneumonia, hemorrhage, shock, inappropriate treatment, leukocytosis (leukocyte count >20000/mm3), thrombocytopenia (platelet count <50000/mm3), hyperbilirubinemia (serum total bilirubin >18 µmol/L), ICU stay, rapidly fatal or ultimately fatal disease, existence of poly-microorganisms, and unknown portal of entry.7,12,29,30,31 Previous studies reports have directly investigated the association between chronic, fatal conditions and S. marcescens-associated bacteremia.12,31 Watanakunakorn reported that both rapidly fatal and ultimately fatal diseases influenced the rate of mortality in S. marcescens bacteremia-related patients with no underlying conditions. Similar results were obtained by Choi, et al.12 with rapidly fatal or ultimately fatal diseases serving as independent prognostic factors for S. marcescens bacteremia-associated fatality. However, in our study, underlying diseases were not significantly different between the fatal and the non-fatal group.
Herein, we identified significant associations between 28-day all-cause mortality and decreased serum albumin, and elevated SOFA score. Serum albumin level was significantly associated with mortality in S. marcescens bacteremia. Hypoalbuminemia was shown in the fatal group although both groups showed evidence of decreased albumin level. Serum albumin levels are used to gauge the general health of a patient, since significant fluctuations are seen during acute illnesses due to changes in vascular permeability and redistribution of fluids.32,33 Moreover, hypoalbuminemia can alter pharmacokinetics (PK) and pharmacodynamics of certain antimicrobial agents.34 Hypoalbuminemia influences PK as a result of decreased binding of the antimicrobial compound to albumin, leading to an increase in the unbound fraction. The relationship between hypoalbuminemia and mortality in acutely ill patients is well established.32,33 Herrmann, et al.32 found that subjects with low serum albumin levels had a higher rate of mortality than the subjects with normal concentrations. The impact of these changes has since been quantified, with mortality risk increasing 137% with each 1 mg/dL decline in serum albumin level.33
Elevated SOFA score was also found to be an independent risk factor for mortality in S. marcescens bacteremia. The SOFA score is a grading system that describes the severity of a patient's illness based on the degree of organ dysfunction, and serves as a useful tool for predicting mortality in bacteremic patients.35 An elevated SOFA score is indicative of severe organ dysfunction and poor prognosis. Several studies demonstrated correlations between SOFA score and clinical outcomes, such as severe sepsis and septic shock, in patients with bacteremia.35,36,37
In our study, the fatal group showed a poorer general condition, including a greater presence of indwelling CVC, urinary catheter, and the onset of bacteremia during the ICU stay than the non-fatal group. These conditions might result in decreased serum albumin level and low SOFA score.
The rate of resistance to cefotaxime (46.9%) during this study period was slightly lower than previous investigations in South Korea.12,38 In our study, there was no statistically significant difference between the fatal and non-fatal group for the presence of 3rd cephalosporin resistance. Most of the patients received appropriately definitive therapies, which could have affected the result.
Our study has some limitations. First, patients with S. marcescens bacteremia included in this study were enrolled from a single center. Second, there is potential for bias and inaccurate data collection due to retrospective nature of this study. Moreover, evidence of a high proportion (>20%) of polymicroorganisms other than S. marcescens may create a bias when analyzing the data. Further prospective studies, conducted in larger patient populations involving multiple centers, are necessary to more accurately identify the risk factors associated with mortality in S. marcescens bacteremia. Finally, the small sample size of those with S. marcescens bacteremia may possibly influence our results.
In conclusions, lower serum albumin level and an elevated SOFA score were significantly associated with adverse outcomes in patients with S. marcescens bacteremia.
Figures and Tables
Fig. 1
Annual distribution of S. marcescens bacteremia in Severance Hospital between January 2006 and December 2012.

Table 1
Clinical Characteristics of the Patients with Serratia marcescens Bacteremia

Table 2
Risk Factors for the Mortality in Patients with S. marcescens Bacteremia
References
1. Eisenstein BI, Zaleznik DF. Enterobacteriaceae. In : Mandell GL, Douglas RG, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia: Churchill Livingstone;2000. p. 2297–2310.
2. Yu VL. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979; 300:887–893.
3. Henjyoji EY, Whitson TC, Oashi DK, Allen BD. Bacteremia due to Serratia marcescens. J Trauma. 1971; 11:417–421.

4. Luzzaro F, Perilli M, Migliavacca R, Lombardi G, Micheletti P, Agodi A, et al. Repeated epidemics caused by extended-spectrum beta-lactamase-producing Serratia marcescens strains. Eur J Clin Microbiol Infect Dis. 1998; 17:629–636.

5. Bonnet R, Sampaio JL, Chanal C, Sirot D, De Champs C, Viallard JL, et al. A novel class A extended-spectrum beta-lactamase (BES-1) in Serratia marcescens isolated in Brazil. Antimicrob Agents Chemother. 2000; 44:3061–3068.

6. Ivanova D, Markovska R, Hadjieva N, Schneider I, Mitov I, Bauernfeind A. Extended-spectrum beta-lactamase-producing Serratia marcescens outbreak in a Bulgarian hospital. J Hosp Infect. 2008; 70:60–65.

7. Saito H, Elting L, Bodey GP, Berkey P. Serratia bacteremia: review of 118 cases. Rev Infect Dis. 1989; 11:912–920.

8. Wong WW, Wang LS, Cheng DL, Lin SJ, Chin TD, Hinthorn DR, et al. Serratia marcescens bacteremia. J Formos Med Assoc. 1991; 90:88–93.
9. Yu WL, Lin CW, Wang DY. Serratia marcescens bacteremia: clinical features and antimicrobial susceptibilities of the isolates. J Microbiol Immunol Infect. 1998; 31:171–179.
10. Shih HI, Lee HC, Lee NY, Chang CM, Wu CJ, Wang LR, et al. Serratia marcescens bacteremia at a medical center in southern Taiwan: high prevalence of cefotaxime resistance. J Microbiol Immunol Infect. 2005; 38:350–357.
11. Cheong HS, Ko KS, Kang CI, Chung DR, Peck KR, Song JH. Clinical significance of infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae blood isolates with inducible AmpC β-lactamase. Microb Drug Resist. 2012; 18:446–452.

12. Choi SH, Kim YS, Chung JW, Kim TH, Choo EJ, Kim MN, et al. Serratia bacteremia in a large university hospital: trends in antibiotic resistance during 10 years and implications for antibiotic use. Infect Control Hosp Epidemiol. 2002; 23:740–747.

13. Engel HJ, Collignon PJ, Whiting PT, Kennedy KJ. Serratia sp. bacteremia in Canberra, Australia: a population-based study over 10 years. Eur J Clin Microbiol Infect Dis. 2009; 28:821–824.

14. Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998; 26:1793–1800.

15. Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Oh MD, et al. Bloodstream infections caused by Enterobacter species: predictors of 30-day mortality rate and impact of broad-spectrum cephalosporin resistance on outcome. Clin Infect Dis. 2004; 39:812–818.

16. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002; 137:791–797.

17. Marik PE, Lipman J. The definition of septic shock: implications for treatment. Crit Care Resusc. 2007; 9:101–103.
18. World Health Organization. International statistical classification of diseases and related health problems. 10th revision. 2nd ed. Geneva: World Health Organization;2004.
19. Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003; 115:529–535.

20. McGregor JC, Rich SE, Harris AD, Perencevich EN, Osih R, Lodise TP Jr, et al. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis. 2007; 45:329–337.

21. Song SW, Kim KT, Ku YM, Park SH, Kim YS, Lee DG, et al. Clinical role of interstitial pneumonia in patients with scrub typhus: a possible marker of disease severity. J Korean Med Sci. 2004; 19:668–673.

22. Carratalà J, Rosón B, Fernández-Sabé N, Shaw E, del Rio O, Rivera A, et al. Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. Eur J Clin Microbiol Infect Dis. 2003; 22:151–157.

23. Clinical and Laboratory Standards Institue. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement M100-S21. Wayne, PA, USA: CLSI;2012.
24. Haddy RI, Mann BL, Nadkarni DD, Cruz RF, Elshoff DJ, Buendia FC, et al. Nosocomial infection in the community hospital: severe infection due to Serratia species. J Fam Pract. 1996; 42:273–277.
25. Laupland KB, Parkins MD, Gregson DB, Church DL, Ross T, Pitout JD. Population-based laboratory surveillance for Serratia species isolates in a large Canadian health region. Eur J Clin Microbiol Infect Dis. 2008; 27:89–95.

26. Park YJ, Park SY, Oh EJ, Park JJ, Lee KY, Woo GJ, et al. Occurrence of extended-spectrum beta-lactamases among chromosomal AmpC-producing Enterobacter cloacae, Citrobacter freundii, and Serratia marcescens in Korea and investigation of screening criteria. Diagn Microbiol Infect Dis. 2005; 51:265–269.

27. Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011; 17:1791–1798.
28. Kim SY, Shin J, Shin SY, Ko KS. Characteristics of carbapenem-resistant Enterobacteriaceae isolates from Korea. Diagn Microbiol Infect Dis. 2013; 76:486–490.

29. Arribas JR, Dominguez A, Folgueira MD, Peña P, Luengo S, Peña JM, et al. Prognostic factors in Serratia bacteremia. Rev Infect Dis. 1990; 12:563–564.

30. Ho PL, Shek RH, Chow KH, Duan RS, Mak GC, Lai EL, et al. Detection and characterization of extended-spectrum beta-lactamases among bloodstream isolates of Enterobacter spp. in Hong Kong, 2000-2002. J Antimicrob Chemother. 2005; 55:326–332.

31. Watanakunakorn C. Serratia bacteremia: a review of 44 episodes. Scand J Infect Dis. 1989; 21:477–483.

32. Herrmann FR, Safran C, Levkoff SE, Minaker KL. Serum albumin level on admission as a predictor of death, length of stay, and readmission. Arch Intern Med. 1992; 152:125–130.

33. Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003; 237:319–334.
34. Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet. 2011; 50:99–110.

35. Routsi C, Pratikaki M, Sotiropoulou C, Platsouka E, Markaki V, Paniara O, et al. Application of the sequential organ failure assessment (SOFA) score to bacteremic ICU patients. Infection. 2007; 35:240–244.

36. Anami EH, Grion CM, Cardoso LT, Kauss IA, Thomazini MC, Zampa HB, et al. Serial evaluation of SOFA score in a Brazilian teaching hospital. Intensive Crit Care Nurs. 2010; 26:75–82.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download