Abstract
Purpose
To evaluate the diagnostic utility and predictors for determinate results of an enzyme-linked immunospot assay using induced sputum cells (IS ELISPOT) for a rapid diagnosis of pulmonary tuberculosis (TB).
Materials and Methods
Subjects suspected of pulmonary TB who had either sputum acid fast bacilli smear-negative or not producing sputum spontaneously were prospectively enrolled. ELISPOT assay was performed using cells from induced sputum.
Results
A total of 43 subjects, including 25 with TB (TB group) and 18 with non-TB disease (non-TB group) were enrolled. Results of IS ELISPOT were determinate in only 17/43 (39%) subjects, but all of determinate results were consistent with the final diagnosis. Of the 43 sputum samples, 11 (26%) were inadequate to perform IS ELISPOT. Of 32 adequate sputum samples, the proportion of determinate results was significantly higher in the TB group (75%, 15/20) than in the non-TB group (17%, 2/12) (p=0.002). The status of active TB was a unique predictor but smear positivity was not a significant predictor for determinate results. In addition, sensitivity of IS ELISPOT (75%, 9/12) in smear negative TB was higher than that of TB-polymerase chain reaction (25%, 3/12).
Rapid diagnosis of pulmonary tuberculosis (TB) is often hindered due to the limitations of the current standard diagnostic tools such as smear and culture. Based on the report that Mycobacterium tuberculosis (MTB)-specific lymphocytes are concentrated at the site of infection in active TB,1,2,3 the feasibility of immunodiagnosis using cells from the site of infection has been evaluated for a rapid diagnosis of TB.4,5,6,7 Several previous studies on enzyme linked immunospot (ELISOPT) assays using bronchoalveolar lavage mononuclear cells have indicated the potential of this assay as a rapid and accurate diagnostic test of TB.8,9,10,11
Sputum induction is an alternative technique for obtaining pulmonary samples. Compared to bronchoscopy, it provides a comparable microbiological yield and is less invasive, less expensive, and has fewer adverse events.12,13 Although ELI-SPOT assay using induced sputum cells (IS ELISPOT) is expected to be a promising tool for rapid diagnosis of TB,14 two previous studies using a commercial ELISPOT assay showed a high rate of inconclusive results, thus indicating it as unfeasible for clinical practice.15,16
Currently, there have been still limited experiences with clinical performance of IS ELISPOT. Therefore, the feasibility of this assay should be reevaluated through more studies in different settings and identification of predictors would be necessary to minimize inconclusive results. The purpose of this study was to evaluate the diagnostic utility and predictors for determinate results of the IS ELISPOT.
Subjects being investigated for pulmonary TB who were unable to produce sputum spontaneously or had one sample of spontaneously produced sputum that was smear-negative for acid fast bacilli (AFB) were prospectively enrolled at Pusan National University Yangsan Hospital between March 2010 and February 2011. No subject had started antituberculosis medication at the time of sputum induction.
Subjects were assigned into the TB group when 1) MTB was cultured in induced or spontaneously produced sputum or 2) there was clear evidence for a clinical diagnosis based on radiology with an appropriate response to treatment. Subjects with negative MTB cultures and an alternative diagnosis were assigned to the non-TB group. All subjects were followed up for at least 6 months after the final diagnosis
The protocol for this study was approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (approval number: 02-2010-21) and each patient provided written informed consent prior to enrollment in the study.
Sputum induction was performed using 3% hypertonic saline solution generated by an ultrasonic nebulizer (Devilbiss Ultraneb 99; Sunrise Medical, Somerset, PA, USA) for 20 minutes in a room with negative-pressure ventilation. All subjects were pre-medicated with 200 µg of salbutamol via a metered-dose inhaler 30 minutes prior to sputum induction. Peak expiratory flow rate (PEFR) was measured every 5 minutes and induction was terminated if PEFR declined by 20%, or if major adverse events were reported. At 5-minute intervals, subjects were asked to clear saliva from their mouth and then expectorate sputum. A trained nurse supervised the entire process. An inadequate induced sputum sample was defined by the following criteria: sputum induction tolerated for <5 minutes, total cells isolated from sputum <1×106, or squamous cell percentage >80%.
Collected induced sputum was processed within 3 hours of sampling. Approximate 3 mL of sputum was removed for microbiological examination including AFB smear, TB culture using Lowenstein-Jensen media, and TB polymerase chain reaction (PCR) (Advansure TB/NTM, LG Life Sciences, Seoul, Korea). Then, remaining sputum was processed for cell isolation. Briefly, after adding equal volume of working sputasol solution (sputasol:PBS, 1:9) (Sputasol, Oxoid SR 0233A), the sample was maintained at room temperature for 20 minutes, during which the sticky materials were frequently dissolved with a sterile empty disposable pipette and a vortex mixer. The sample was filtered through a 40-µm nylon mesh into another conical tube. After centrifugation (1500×g, 10 minutes), the supernatants were collected and Ficoll-gradient centrifugation of the pellet was performed to isolate the leukocytes. It was then washed twice with RPMI 1640 medium (Gibco; Gaithersburg, MD, USA), and the isolated cells were counted with a hemocytometer and cell viability was assessed using trypan blue. Counted cells were used for the preparation of cytospin slides and for the ELISPOT assay.
Cytospin slides were stained with Hemacolor (Merck; Darmstadt, Germany). Differential cell counts were performed under light microscope using a set of standardized morphometric appearance criteria (magnification, ×400), and the percentage of each cell population, including neutrophils, macrophages, lymphocytes, and bronchial epithelial cells, was individually recorded after counting more than 200 cells. And absolute cell numbers were calculated with total cell counts.
The ELISPOT assay (T SPOT-TB; Oxford Immunotec, Oxford, UK) was performed according to the manufacturer's instructions. This assay kit included nil control, early secretory antigen target (ESAT)-6-derived peptide (Panel A), CFP-10-derived peptide (Panel B), and phytohaemagglutinin in positive control wells. Briefly, isolated sputum cells were plated at 2.5×105 cells/well in 96-well T-spot plates. After incubating the plate overnight in a humidified incubator at 37℃ and 5% CO2, the plate was washed 3 times with fresh PBS solution and Conjugate Reagent solution was added. After incubating at 4℃ for 1 hour, the plate was washed 3 times with fresh PBS solution and substrate solution was added. The plate was incubated at room temperature for 7 minutes and wash with distilled water to stop the reaction. Then, the plate was allowed to dry and the spots were counted with Immuno.spot (Cellular Technology Ltd., Cleveland, OH, USA).
As per the manufacturer's instructions for ESAT-6 and CFP-10, a test was scored as qualitatively reactive if either (a) for nil control of 0-5 spots, the antigen well spot count minus the nil control was ≥6 or (b) for nil control of >5 spots, the antigen count was ≥2 times the nil control. Overall, a T SPOT-TB test was considered reactive if either ESAT-6 or CFP-10 was reactive. The results were considered as indeterminate when <20 spots were found in positive control well (positive control failure) or when >5 spots in the negative control well and indicated non-reactive after calculating with the spots in antigen-wells (negative control failure).
The chi-square test or Fisher's exact test was performed to compare categorical variables, and Student's t-test or Mann-Whitney test was performed to compare continuous variables. Simple logistic regression analysis was used to assess univariate associations between tested variables and IS ELISPOT result (determinate vs. indeterminate). Correlation analysis between variables was carried out by Spearman's rho test. All analysis were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA), and the results with p<0.05 were considered statistically significant.
A total of 43 subjects were enrolled in the study. Thirty one of them (72%) had AFB smear negative sputum and 12 (28%) did not produce sputum spontaneously. Among 43 subjects, 25 (58%) were assigned to the TB group and 18 (42%) to the non-TB group. In the TB group, 20 of 25 subjects had MTB-positive cultures from their induced sputum samples, while 13 of those 20 subjects were AFB smear positive. TB-PCR was positive in 12 of 13 smear positive and 3 of 12 smear negative samples. Final diagnosis of the 18 subjects in non-TB group included: former TB without reactivation (n=7), pneumonia (n=4), bronchiectasis (n=2), lung cancer (n=2), nontuberculous mycobacterial pulmonary disease (n=2), and idiopathic pulmonary fibrosis (n=1).
Of the 43 subjects, 12 (28%) had at least 1 comorbidity; malignancy was the most common (n=7: lung, n=2; stomach, n=2; colon, n=2; and kidney, n=1), followed by psychiatric disease (n=2), cardiovascular disease (n=1), gastrointestinal disease (n=1), and diabetes mellitus (n=1). HIV-ELISA tests were performed on 2 patients and both were negative.
There were no significant adverse events or PEFR reduction to terminated premature sputum induction. The median volume of induced sputum processed for cell isolation was 7.5 mL (range, 2-18) in the TB group and 7.5 mL (range, 3-18) in the non-TB group (p=0.992). The median value of total cell numbers obtained from induced sputum was 3.1×106 cells (range, 0.2-35.0) in the TB group and 2.2×106 cells in the non-TB group (range, 0.6-19.6) (p=0.808).
Of 43 sputum samples, 11 (26%) were inadequate to perform the ELISPOT assay. Eight samples had insufficient total cell numbers and 3 samples had >80% of squamous epithelial cells. The proportion of inadequate sputum was not significantly different between the TB (20%, 5/25) and non-TB group (33%, 6/18) (p=0.480) (Table 1).
The median number of total cells from adequate sputum was 3.3×106 cells (range, 1.0-3.5), which was significantly higher than the value of 0.9×106 cells (range, 0.2-1.4) from inadequate sputum (p=0.034). There was no correlation between total cell numbers and volumes of sputum (Spearman's rho=0.108, n=43, p=0.497). In addition, there were no significant differences in the tested variables such as sputum volume, gender, age, diagnosis of TB, smoking history, and underlying diseases between the adequate and inadequate samples (data not shown).
Cytospin slides were obtained from induced sputum cells, and differential cell counts were performed. Fig. 1 shows the percentage of each cell type. Alveolar macrophages were predominant in the TB group while neutrophil numbers were higher than any other cell types in the non-TB group. The percentage of lymphocytes was significantly higher in the TB group than that in the non-TB group, whereas the percentage of neutrophils was inverse between the groups.
IS ELISPOT was performed on 32 adequate sputum samples after excluding 11 inadequate samples. The median numbers of ESAT-6- and CFP-10-specific cells were higher in the TB group (24; range, 0-409, and 37; 0-215, respectively) compared to the non-TB group (11; 0-86, and 13; 0-112, respectively), but the differences were not statistically significant (p=0.095, and 0.184, respectively). However, after subtraction of spots from the negative controls, the median number of ESAT-6- and CFP-10-specific cells were significantly higher in the TB group (15; 0-210, and 20; 0-200, respectively) than in the non-TB group (0; 0-5 and 0; 0-3, p<0.001 and p<0.001, respectively) (Fig. 2).
Of 32 adequate sputum samples, 17 showed determinate results whereas 15 were indeterminate. The proportion of determinate results was higher in the TB (75%, 15/20) than in the non-TB group (17%, 2/12) (p=0.002), and all of these determinate results were consistent with the final diagnosis. The characteristics of these 17 subjects are shown in Table 2, and the reasons for inconclusive results are explained in Table 1.
Sensitivities of TB-PCR (60%, 15/25) and IS ELISPOT (60%, 15/25) in the TB group were same. However, in 12 AFB smear-negative samples, the sensitivity of IS ELISPOT (75%, 9/12) was much higher than that of TB-PCR (25%, 3/12).
Among the 32 subjects with adequate sputum, univariate analysis indicated the status of active TB as a unique predictor for determinate results (odds ratio, 15.0; 95% confidence interval, 2.42-93.01; p=0.003) (Table 3). In contrast, as for the TB group of 20 subjects with adequate sputum, no significant predictor for determinate results was observed. Unexpectedly, even smear positivity and extent of chest radiography (CXR) were not significant factors (Table 4).
In the present study, we investigated the diagnostic utility of the IS ELISPOT for pulmonary TB, and found that the proportion of determinate results obtained by using the IS ELISPOT was only 39% (17/43); however, all of these determinate results were consistent with the final diagnosis. The proportion of determinate results was significantly higher in the TB group than in the non-TB group, and the active status of TB was a unique predictor for determinate result. When adequate sputum is obtained in the TB group, the proportion of determinate results was relatively high (75%, 15/20) and smear positivity was not a significant predictor for determinate results. In addition, sensitivity of IS ELISPOT (75%, 9/12) was higher than that of TB-PCR (25%, 3/12) in smear-negative samples, suggesting that IS ELISPOT may increase diagnostic accuracy for smear-negative TB when smear microscopy is unhelpful.
Two studies have reported the diagnostic utility of the IS ELISPOT assay for pulmonary TB.15,16 The major common limitation of the IS ELISPOT assay in their studies was the high rate of inconclusive results, as shown in the present study. There are 2 main reasons for the inconclusive results: 1) inadequate sputum samples associated with the procedures of sputum induction and sputum processing for cell isolation, and 2) indeterminate results associated with ELISPOT assay related factors.
In our study, diagnostic yield of IS ELISPOT was quite different between TB and non-TB groups. This difference seems to have resulted from the factors associated with current ELISPOT assay rather than those associated with the procedures of sputum induction and processing. Although the proportion of inadequate sputum samples was not significantly different between TB and non-TB groups, the rate of indeterminate results was significantly higher in non-TB group (56%) than in TB group (20%). This finding suggests that the current format of IS ELISPOT may have major limitation to be a rule-out test for TB, consistent with previous IS ELISPOT studies.15,16
In our experiments, we were able to exclude certain minor reasons for inconclusive results such as homogenization failure, bacterial and fungal colonization, and inadequate volume of sputum that have been mentioned in the previous studies.15,16 In the present study, a trained nurse supervised the entire process of sputum induction; this might have contributed to the lower rate of inadequate sputum samples compared to previous studies. Indeed, proper supervision during sputum induction has been shown to resul in higher diagnostic yield in microbiologic conformation.17 These findings suggest the possibility of improving the diagnostic yield of IS ELISPOT through improvement and standardization of sputum induction and processing.
An important finding of this study was that smear and culture positivity and extent of CXR were not predictors for determinate results, suggesting that the number of antigen-specific T cells in the airway of pulmonary TB patients may not directly correlate with the burden of MTB bacilli. Condos, et al.18 reported earlier that patients with advanced TB had lower lymphocyte numbers and IFN-γ levels in the involved lobe than those with less advanced TB. Another finding in the present study was that diagnostic yield of IS ELISPOT was comparable to that of TB-PCR in TB group and much higher in AFB smear-negative samples. This is consistent with the recent report that local immunodiagnosis using T-SPOT on bronchoalveolar lavage cells may increase diagnostic accuracy where clinical suspicion of pulmonary TB persists despite a negative Xpert result.19 These findings suggest the potential of IS ELISPOT as an adjunctive test for rapid diagnosis of smear negative TB, although IS ELISPOT by itself is not expected to play a critical role in the diagnosis of TB. Nevertheless, further studies are needed to improve the assay formats and technologies.
This study has several limitations. First, the ELISPOT assay was not performed on peripheral blood mononuclear cells (PBMCs) as controls. It has clearly been demonstrated that ELISOPT assays on PBMCs have limited utility for diagnosing active TB. However, the parameters of compartmentalization of immune response such as the ratio of MTB-specific lymphocytes derived from the site of the infection and the peripheral blood have not been evaluated for the predictor for determinate result of IS ELISPOT assay. Second, the number of subjects involved in the study might be too small to optimally evaluate predictors for determinate results. However, this study has the highest number of determinate results among all IS ELISPOT studies reported so far.
In conclusion, IS ELISPOT showed relatively high diagnostic yield and accuracy in TB group, independent of smear positivity. Therefore, IS ELISPOT may provide additional diagnostic yields to microbiological tools for rapid diagnosis for smear-negative TB.
Figures and Tables
 | Fig. 1Differential cell counts in induced sputum cells. The adequate sputum samples for 20 TB and 12 non-TB subjects were evaluated. Results are presented as percentage of each cell type. Horizontal lines indicate median values. The difference of each cell type between TB and non-TB group is shown only when it is statistically significant (p<0.05). TB, tuberculosis. |
 | Fig. 2Concentration of interferon-gamma releasing cells reactive to early secretory antigenic target (ESAT)-6 and culture filtrate protein (CFP)-10 measured using enzyme-linked immunospot assay with induced sputum cells in study subjects (n=32). All spots are documented after subtraction of spots in negative control. A value of 0 was assigned when the antigen well spot count minus the nil control was <0. Horizontal lines show median values. TB, tuberculosis. |
Table 1
Performance of Enzyme-Linked Immunospot Assay Using Induced Sputum Cells
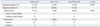
Table 2
Characteristics of 17 Subjects with the Determinate Result of Enzyme-Linked Immunospot Assay Using Induced Sputum Cells
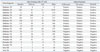
Table 3
Predictors for Determinate Result among 32 Subjects with Adequate Induced Sputum
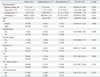
Table 4
Predictors for Determinate Result among 20 Tuberculosis Patients with Adequate Induced Sputum

ACKNOWLEDGEMENTS
This work was supported by Medical Research Institute Grant (2010-17), Pusan National University, Republic of Korea.
References
1. Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993; 61:3482–3489.

2. Wilkinson KA, Wilkinson RJ, Pathan A, Ewer K, Prakash M, Klenerman P, et al. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin Infect Dis. 2005; 40:184–187.

3. Nemeth J, Winkler HM, Zwick RH, Rumetshofer R, Schenk P, Burghuber OC, et al. Recruitment of Mycobacterium tuberculosis specific CD4+ T cells to the site of infection for diagnosis of active tuberculosis. J Intern Med. 2009; 265:163–168.

4. Losi M, Bossink A, Codecasa L, Jafari C, Ernst M, Thijsen S, et al. Use of a T-cell interferon-gamma release assay for the diagnosis of tuberculous pleurisy. Eur Respir J. 2007; 30:1173–1179.

5. Thomas MM, Hinks TS, Raghuraman S, Ramalingam N, Ernst M, Nau R, et al. Rapid diagnosis of Mycobacterium tuberculosis meningitis by enumeration of cerebrospinal fluid antigen-specific T-cells. Int J Tuberc Lung Dis. 2008; 12:651–657.
6. Kösters K, Nau R, Bossink A, Greiffendorf I, Jentsch M, Ernst M, et al. Rapid diagnosis of CNS tuberculosis by a T-cell interferon-gamma release assay on cerebrospinal fluid mononuclear cells. Infection. 2008; 36:597–600.

7. Cho OH, Park KH, Park SJ, Kim SM, Park SY, Moon SM, et al. Rapid diagnosis of tuberculous peritonitis by T cell-based assays on peripheral blood and peritoneal fluid mononuclear cells. J Infect. 2011; 62:462–471.

8. Jafari C, Ernst M, Kalsdorf B, Greinert U, Diel R, Kirsten D, et al. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am J Respir Crit Care Med. 2006; 174:1048–1054.

9. Jafari C, Ernst M, Strassburg A, Greinert U, Kalsdorf B, Kirsten D, et al. Local immunodiagnosis of pulmonary tuberculosis by enzyme-linked immunospot. Eur Respir J. 2008; 31:261–265.

10. Jafari C, Thijsen S, Sotgiu G, Goletti D, Domínguez Benítez JA, Losi M, et al. Bronchoalveolar lavage enzyme-linked immunospot for a rapid diagnosis of tuberculosis: a Tuberculosis Network European Trialsgroup study. Am J Respir Crit Care Med. 2009; 180:666–673.

11. Dheda K, van Zyl-Smit RN, Meldau R, Meldau S, Symons G, Khalfey H, et al. Quantitative lung T cell responses aid the rapid diagnosis of pulmonary tuberculosis. Thorax. 2009; 64:847–853.

12. McWilliams T, Wells AU, Harrison AC, Lindstrom S, Cameron RJ, Foskin E. Induced sputum and bronchoscopy in the diagnosis of pulmonary tuberculosis. Thorax. 2002; 57:1010–1014.

13. Brown M, Varia H, Bassett P, Davidson RN, Wall R, Pasvol G. Prospective study of sputum induction, gastric washing, and bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in patients who are unable to expectorate. Clin Infect Dis. 2007; 44:1415–1420.

14. Breen RA, Hardy GA, Perrin FM, Lear S, Kinloch S, Smith CJ, et al. Rapid diagnosis of smear-negative tuberculosis using immunology and microbiology with induced sputum in HIV-infected and uninfected individuals. PLoS One. 2007; 2:e1335.

15. Cashmore TJ, Peter JG, van Zyl-Smit RN, Semple PL, Maredza A, Meldau R, et al. Feasibility and diagnostic utility of antigen-specific interferon-gamma responses for rapid immunodiagnosis of tuberculosis using induced sputum. PLoS One. 2010; 5:e10389.
16. Dilektasli AG, Erdem E, Durukan E, Eyüboğlu FO. Feasibility of the T-SPOT.TB assay for the immunodiagnosis of smear-positive pulmonary tuberculosis using induced sputum. Int J Tuberc Lung Dis. 2010; 14:1205–1208.
17. Chang KC, Leung CC, Yew WW, Tam CM. Supervised and induced sputum among patients with smear-negative pulmonary tuberculosis. Eur Respir J. 2008; 31:1085–1090.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download