Abstract
Purpose
The identification of sick sinus syndrome (SSS) in patients with atrial flutter (AFL) is difficult before the termination of AFL. This study investigated the patient characteristics used in predicting a high risk of SSS after AFL ablation.
Materials and Methods
Out of 339 consecutive patients who had undergone radiofrequency ablation for AFL from 1991 to 2012, 27 (8%) had SSS (SSS group). We compared the clinical characteristics of patients with and without SSS (n=312, no-SSS group).
Results
The SSS group was more likely to have a lower body mass index (SSS: 22.5±3.2; no-SSS: 24.0±3.0 kg/m2; p=0.02), a history of atrial septal defects (ASD; SSS: 19%; no-SSS: 6%; p=0.01), a history of coronary artery bypass graft surgery (CABG; SSS: 11%; no-SSS: 2%; p=0.002), and a longer flutter cycle length (CL; SSS: 262.3±39.2; no-SSS: 243.0±40; p=0.02) than the no-SSS group. In multivariate analysis, a history of ASD [odds ratio (OR) 3.7, 95% confidence interval (CI) 1.2-11.4, p=0.02] and CABG (7.1, 95% CI 1.5-32.8, p=0.01) as well as longer flutter CL (1.1, 95% CI 1.0-1.2, p=0.04) were independent risk factors for SSS.
Atrial flutter (AFL) is the second most common atrial tachyarrhythmia after atrial fibrillation. Catheter ablation for typical AFL has been well established and ensures a high success rate and adequate safety.1 However, sick sinus syndrome (SSS) is occasionally uncovered when long-term, persistent AFL is terminated by ablation.2,3 Permanent pacemaker implantation is necessary in these cases. Sinus node function cannot be assessed during AFL, and preoperative prediction of underlying SSS has not yet been investigated. Currently, there is limited data on this issue.
The purpose of this study is to investigate the predictors of SSS before AFL ablation in patients with persistent AFL.
The patient population included 339 consecutive patients who had undergone radiofrequency catheter ablation for AFL at Severance Cardiovascular Hospital from January 1996 to May 2012. Patients were enrolled retrospectively in a registry. Inclusion criteria were as follows: 1) cavotricuspid isthmus (CTI) participation in the arrhythmic circuit confirmed by an EP study, 2) an atrial activation pattern during atrial tachycardia showing a clockwise or counterclockwise rotation around the tricuspid annulus (TA), 3) termination of the AFL by CTI linear ablation, and 4) atypical flutter involving the atriotomy.
Typical AFL was diagnosed when a surface ECG showed flutter waves that were predominantly negative in leads II, III, and aVF and positive in lead V1, with a regular atrial rate. Also enrolled were patients with a surface ECG uncharacteristic of typical AFL as well as those with typical AFL whose atrial tachyarrhythmias were confirmed to have arrhythmic circuits including the CTI and tricuspid annulus. Patients were excluded if they had previously undergone AFL ablation or implantation of a pacemaker for SSS.
Patient histories, baseline characteristics, procedural data, and follow-up information were collected by a retrospective query of all records within a computerized patient record system. The database contained information about patient demographics and preprocedural risk factors, including age, body mass index, congestive heart failure, hypertension, diabetes mellitus, prior transient ischemic attack or stroke, dyslipidemia, coronary artery disease, vascular disease, congenital heart disease, cardiomyopathy, and preprocedure atrial fibrillation (AF). Preprocedure echocardiograms were reviewed to determine the left ventricular ejection fraction, left atrial size, left atrial volume index, and E/E'. The use of medications, including antiplatelet drugs (e.g., aspirin, clopidogrel), warfarin, and antiarrhythmic drugs was documented as well.
All antiarrhythmic agents were ceased ≥5 half-lives before the electrophysiological (EP) procedure, and a signed consent form was obtained from each patient. Electrophysiological studies were performed in the postabsorptive state. A duodecapolar catheter with 2-5-2 mm interelectrode spacing (St. Jude Medical, St. Paul, MN, USA) was positioned in the right atrium (RA), parallel to the TA so that the distal pole was located in the medial region of the CTI. A decapolar catheter was inserted within the coronary sinus (CS), with the proximal bipole located at the ostium. Quadripolar catheters were positioned at the His bundle and RA. Then, a non-irrigated ablation catheter with a 4 or 8 mm tip was placed close to the right ventricle (RV), within the CTI. Bipolar intracardiac electrograms that were filtered between 30 and 500 Hz were digitally recorded and stored simultaneously with a 12-lead surface ECG.
The flutter cycle length (CL) was preliminarily calculated as an average over 10 consecutive cycles in lead V1 by a surface ECG performed the day before the ablation procedure. In the same fashion, the flutter CL was measured at the proximal CS 10 min after insertion of the sheaths without any sedation so that the calculated value would not be affected by changes in autonomic tone resulting from venipuncture or sedative drugs. The ventricular cycle length during AFL was measured with a surface ECG in the same manner. Cavotricuspid isthmus dependence was confirmed if concealed entrainment was identified when pacing the CTI4,5 and if the difference between the post-pacing interval at the CTI and flutter CL was within 30 ms. A counterclockwise or clockwise activation sequence around the TA during AFL was confirmed by sequential mapping in patients with typical flutter waves and by both sequential mapping and electroanatomic mapping with a CARTO system (Biosense Webster) in patients with atypical flutter waves. Subsequently, a linear lesion was made by continuously applying radiofrequency energy with a temperature target of 60℃ and a power limit of 50 W during a stepwise withdrawal of the ablation catheter from the RV and towards the inferior vena cava. Procedural success was defined as the termination of AFL during the radiofrequency application and the creation of a bidirectional CTI block.
Twenty minutes after successful AFL ablation, baseline intracardiac conduction intervals, including the sinus rhythm cycle length, atrio-His (AH), and His-ventricle (HV) intervals, were measured during sinus rhythm.
Patients received isoproterenol infusion or temporary pacing from a catheter within the RA if they manifested any significant SSS following AFL termination by the catheter ablation. Sick sinus syndrome was defined as a syndrome encompassing a number of sinus node abnormalities, including the following: 1) persistent spontaneous sinus bradycardia not caused by drugs and inappropriate for the physiological circumstance, 2) sinus arrest or exit block, 3) a combination of SA and atrioventricular conduction disturbance, or 4) an alternation of paroxysms of rapid regular or irregular atrial tachyarrhythmias and periods of slow atrial and ventricular rates.6 The indication for temporary pacing included one or more of the following with or without symptoms such as syncope, dizziness and dyspnea: 1) sinus bradycardia with a heart rate of <40 beats/min, 2) sinus arrest, or 3) repeated sinus pauses longer than 3 s. Patients who had bradycardia-related symptoms resulting from the above EP abnormalities after the CTI ablation had permanent dual-chamber pacemakers implanted.
Continuous data are expressed as mean±SD, and categorical variables are expressed as counts and percentages (%). Normality tests were performed for each variable to determine whether a data set was well modeled by a normal distribution or not. Univariate comparisons were performed using Student's t-test or the Mann-Whitney U test for continuous variables and a chi-square test or Fisher's test for categorical variables. A multivariate analysis was performed to identify predictors associated with sinoatrial node dysfunction. Statistical significance was established at a value of p<0.05. Statistical analysis was performed by SPSS Version 18.0 (SPSS Inc., Chicago, IL, USA).
A total of 350 patients were considered eligible for inclusion; however, 11 patients were excluded from our study due to the following reasons: 6 patients had previous AFL ablations and 5 had previous pacemaker implantations for SSS. Finally, a total of 339 patients (mean age 56±14 years, 73 women) were enrolled in our study. After successful AFL ablation, 27 patients (8%) had SSS and needed isoproterenol infusion or temporary pacing.
The baseline characteristics of patients with or without SSS are summarized in Table 1. In 37 out of 329 patients (11.2%), AFL was combined with AF. The prevalence of AF between two groups revealed no statistical difference (SSS: 11.1%; no-SSS: 11.3%; p=0.98). Patients with SSS were more likely to have a lower body mass index (SSS: 22.5±3.2 kg/m2; no-SSS: 24.0±3.0 kg/m2; p=0.02), a history of atrial septal defect (ASD; SSS: 19%; no-SSS: 6%; p=0.01), and a history of coronary artery bypass graft surgery (CABG; SSS: 11%; no-SSS: 2%; p=0.002). We observed a trend of higher incidence of congestive heart failure in the SSS group than in the no-SSS group (SSS: 15%; no-SSS: 7%; p=0.31). Among 23 patients (7%) with ASD, 19 patients had secundum ASDs, 3 had primum ASDs, and 1 had a sinus venosus ASD. All patients with SSS had ostium secundum ASDs. While class III antiarrhythmic drugs were more frequently prescribed in patients with no SSS (SSS: 0%; no-SSS: 12%; p<0.001), digitalis was prescribed more frequently in those with SSS (SSS: 22%; no-SSS: 7%; p=0.008).
Table 2 represents the electrophysiological parameters in patients with or without SSS. Patients with SSS were more likely to have atypical flutter waves (SSS: 30%; no-SSS: 3%; p=0.006) and longer flutter CLs (SSS: 262±39 ms; no-SSS: 243±40 ms; p=0.02). QRS duration, sinus rhythm CL, AH interval, and HV interval revealed no statistical differences between the two groups.
In the univariate logistic regression analysis, age >75 years, low body mass index, history of CABG surgery, prior cardiac surgery for ASD, longer flutter CL, and antiarrhythmic drug and digitalis use emerged as predictors of SSS after AFL ablation. In multivariate models, history of CABG surgery [odds ratio (OR) 7.1, 95% confidence interval (CI) 1.5-32.8, p=0.01], history of ASD (OR 3.7, 95% CI 1.2-11.4, p=0.02), and longer flutter CL (OR 1.1, 95% CI 1.0-1.2, p=0.04) were independent predictors of SSS (Table 3).
Out of 27 patients with SSS, 14 had transient SSS with a mean duration of 19.9±23.1 hours after AFL ablation. Thirteen patients had irreversible SSS, including 4 with junctional escape rhythms and 9 with sinus bradycardia. During the 6.0±4.2 years (range: 1-16.1 years) follow up, 8 of 27 patients (30%) required permanent pacemaker implantation. The mean time of pacemaker implantation after AFL ablation was 20.5 days (interquartile range: 15.25-38.25 days). One patient needed a pacemaker at 1 year 7 months after AFL ablation. Patients with irreversible SSS had heart failure more frequently than those with reversible SSS (irreversible: 31%; reversible: 0%; p=0.03). Hypertension (irreversible: 8%; reversible: 50%; p=0.009) and beta blocker use (irreversible: 8%; reversible: 50%; p=0.02) were more common in patients with reversible SSS (Table 4).
In the present study, we evaluated the patient characteristics that predicted a high risk of SSS after successful AFL ablation. In observing the 8% of patients who had SSS after successful AFL ablations, we found that a history of cardiac surgery for ASD and CABG as well as a longer flutter CL increased the risk of SSS. Half of SSS cases after AFL ablation were transient and improved within one day. Heart failure was associated with irreversible SSS, and beta blocker use was more common in patients with reversible SSS.
Increased risk of SSS after open-heart surgery has repeatedly been identified in previous studies,7,8 and a similar trend was also observed in the present analysis. Direct damage to the sinus node or sinus node blood supply during cardiac surgery has been suggested as the causal mechanism. We observed a significant trend towards an association between atypical flutter waves and SSS, and this may be causally related to previous open-heart surgery as well. In typical AFL, the sites with conduction delay are located in the CTI and crista terminalis; however, in atypical AFL, other sites with conduction delay exist, likely produced by previous surgical scars.9 History of ASD or CABG surgery itself may be predictors of sinus node dysfunction after atrial flutter ablation. Therefore, it is vital to be able to predict the manifestation of SSS after AFL ablation in these patients.
In this study, the CL of AFL was longer in patients with SSS than those without SSS. The flutter CL is one of the few electrophysiological parameters of the atria obtained from surface ECG during AFL. Based on the well-recognized circus movement theory,10 flutter CL depends on the atrial conduction velocity as well as the length of the circuit. Slowing of the conduction velocity is closely related to SSS in diseased atria and provides an arrhythmogenic substrate causing atrial tachyarrhythmias including AFL.11,12,13,14 Previous studies have shown that subjects with SSS demonstrated diffuse atrial remodeling.11,12,13,14,15 Sanders, et al.12 suggested that a significant derangement of sinus node function would require extensive loss of automatic pacemaker tissue and widespread impairment of sinoatrial conduction. The CLs of AFL and AF were suggested as predictors of SSS.16,17
In this study, half of the patients requiring temporary pacing for significant SSS following termination of AF recovered their sinus node function within one day after the ablation. The recovery of sinus node function after the ablation of tachycardia was previously reported in AFL and atrial fibrillation.2,18 Interestingly, aside from the tachycardia-induced reversible changes in sinus node function, beta blocker usage was associated with transient SSS. Moreover, irreversible SSS was associated with heart failure, possibly because an arrhythmogenic substrate causing the AFL was present in the atrium of the patients with SSS as previously indicated.14,19,20
We enrolled patients with persistent AFL; however, the duration of persistent or chronic AFL may be difficult to define accurately due to potential misinterpretation of symptoms. Second, flutter CL may be affected by autonomic tone.21 To control this confounding effect, autonomic blockade is needed. The anatomical characteristics of the RA can also introduce variability into the AFL CL.22 Drug choice (class III AAD or digitalis) may be related to the physician's choice, and drug treatment may be associated with sinus rate or sinus node function before enrollment or flutter ablation. Although all antiarrhythmic agents were ceased ≥5 half-lives before the EP procedure, amiodarone might have affected the sinus rate and sinus node function because of the long half-life. Finally, this was a retrospective study, and the data were gathered from a non-selected population. Therefore, it is possible that the predictors of SSS requiring pacemaker implantation determined in our study would not be clinically significant enough to be directly applied to clinical practice. Thus, it is desirable to conduct a prospective study including selected populations in the future.
History of cardiac surgery for ASD and CABG as well as longer flutter CL increased the risk of SSS after AFL ablation. This result suggests that flutter CL prior to ablation can be helpful for risk stratification of patients with persistent AFL who may develop SSS requiring a pacemaker implantation. Also, the flutter CL may yield important information regarding atrial remodeling. Finally, heart failure was associated with irreversible SSS.
Figures and Tables
Table 1
Clinical Characteristics of the Study Patients
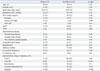
Table 2
Pre- and Post-Ablation Electrophysiological Parameters

Table 3
Predictors of Sick Sinus Syndrome after Univariate and Multivariate Analyses (n=339)
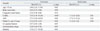
Table 4
Clinical Characteristics of Patients with Irreversible and Reversible SSS
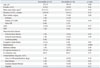
ACKNOWLEDGEMENTS
This study was supported in part by research grants from Yonsei University College of Medicine (8-2011-0250, 7-2011-0758, 7-2011-0702, 7-2011-0015), the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012-0007604, 2012-045367) and a grant from the Korean Healthcare Technology R&D Project funded by the Korean Ministry of Health & Welfare (A121668).
References
1. Blomström-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary. A report of the American college of cardiology/American heart association task force on practice guidelines and the European society of cardiology committee for practice guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol. 2003; 42:1493–1531.
2. Daoud EG, Weiss R, Augostini RS, Kalbfleisch SJ, Schroeder J, Polsinelli G, et al. Remodeling of sinus node function after catheter ablation of right atrial flutter. J Cardiovasc Electrophysiol. 2002; 13:20–24.

3. Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007; 115:1921–1932.
4. Maury P, Duparc A, Hebrard A, El Bayomy M, Delay M. Prevalence of typical atrial flutter with reentry circuit posterior to the superior vena cava: use of entrainment at the atrial roof. Europace. 2008; 10:190–196.

5. Morton JB, Sanders P, Deen V, Vohra JK, Kalman JM. Sensitivity and specificity of concealed entrainment for the identification of a critical isthmus in the atrium: relationship to rate, anatomic location and antidromic penetration. J Am Coll Cardiol. 2002; 39:896–906.

6. Braunwald E, Bonow RO. Braunwald's heart disease: review and assessment. 9th ed. Philadelphia: Saunders;2012.
7. Koplan BA, Stevenson WG, Epstein LM, Aranki SF, Maisel WH. Development and validation of a simple risk score to predict the need for permanent pacing after cardiac valve surgery. J Am Coll Cardiol. 2003; 41:795–801.

8. Onalan O, Crystal A, Lashevsky I, Khalameizer V, Lau C, Goldman B, et al. Determinants of pacemaker dependency after coronary and/or mitral or aortic valve surgery with long-term follow-up. Am J Cardiol. 2008; 101:203–208.

9. Yang Y, Cheng J, Bochoeyer A, Hamdan MH, Kowal RC, Page R, et al. Atypical right atrial flutter patterns. Circulation. 2001; 103:3092–3098.

10. Frame LH, Page RL, Hoffman BF. Atrial reentry around an anatomic barrier with a partially refractory excitable gap. A canine model of atrial flutter. Circ Res. 1986; 58:495–511.

11. Elvan A, Wylie K, Zipes DP. Pacing-induced chronic atrial fibrillation impairs sinus node function in dogs. Electrophysiological remodeling. Circulation. 1996; 94:2953–2960.

12. Sanders P, Morton JB, Kistler PM, Spence SJ, Davidson NC, Hussin A, et al. Electrophysiological and electroanatomic characterization of the atria in sinus node disease: evidence of diffuse atrial remodeling. Circulation. 2004; 109:1514–1522.

13. Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004; 110:897–903.

14. Stiles MK, Wong CX, John B, Kuklik P, Brooks AG, Lau DH, et al. Characterization of atrial remodeling studied remote from episodes of typical atrial flutter. Am J Cardiol. 2010; 106:528–534.

15. Stevenson IH, Kistler PM, Spence SJ, Vohra JK, Sparks PB, Morton JB, et al. Scar-related right atrial macroreentrant tachycardia in patients without prior atrial surgery: electroanatomic characterization and ablation outcome. Heart Rhythm. 2005; 2:594–601.

16. Sairaku A, Nakano Y, Oda N, Makita Y, Kajihara K, Tokuyama T, et al. Prediction of sinus node dysfunction in patients with long-standing persistent atrial fibrillation using the atrial fibrillatory cycle length. J Electrocardiol. 2012; 45:141–147.

17. Sairaku A, Nakano Y, Oda N, Makita Y, Kajihara K, Tokuyama T, et al. Prediction of sinus node dysfunction in patients with persistent atrial flutter using the flutter cycle length. Europace. 2012; 14:380–387.

18. Hocini M, Sanders P, Deisenhofer I, Jaïs P, Hsu LF, Scavée C, et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation. 2003; 108:1172–1175.

19. Anand N, McCrindle BW, Chiu CC, Hamilton RM, Kirsh JA, Stephenson EA, et al. Chronotropic incompetence in young patients with late postoperative atrial flutter: a case-control study. Eur Heart J. 2006; 27:2069–2073.

20. de Groot NM, Schalij MJ. The relationship between sinus node dysfunction, bradycardia-mediated atrial remodelling, and post-operative atrial flutter in patients with congenital heart defects. Eur Heart J. 2006; 27:2036–2037.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download