Although the effects of prolonged corticosteroid treatment on survival in acute respiratory distress syndrome (ARDS) remain controversial, the treatment has shown positive effects on lung mechanics and gas exchanges.
1,
2,
3,
4 In a recent multicenter randomized controlled trial,
4 prolonged administration of low-dose methylprednisolone (1 mg/kg/day) initiated in early ARDS was associated with earlier resolution of pulmonary and extrapulmonary organ dysfunction, as well as reduction in duration of mechanical ventilation (MV) and intensive care unit (ICU) stay. On the other hand, however, prolonged corticosteroid treatment may induce serious adverse events.
5,
6 Therefore, early prediction of response to prolonged corticosteroid treatment would be helpful to decide whether to continue treatment.
We conducted a prospective observational study to evaluate predictors of response to prolonged corticosteroid treatment in early ARDS. Our hypothesis was that response to prolonged corticosteroid treatment was expected within 3 days after the initiation of treatment.
The investigation was conducted from July 2010 through November 2011 in the medical ICU of Samsung Medical Center (a 1961-bed, university-affiliated, tertiary referral hospital in Seoul, Korea). The Institutional Review Board of Samsung Medical Center approved this study. Written informed consent was obtained from patients or their surrogates. Adult patients who were intubated and receiving MV were eligible for enrollment if, within 72 hours after intubation, they met diagnostic criteria for ARDS as previously defined.
7 On the day of study entry, the partial pressure of arterial oxygen (PaO
2)/fraction of inspired oxygen (FiO
2) ratio (PF ratio) had to be less than 200 at positive end-expiratory pressure (PEEP) of 8 cmH
2O or more. Severity of ARDS was classified by the Berlin definition.
8 The exclusion criteria were imminent death, contraindication to corticosteroid treatment, current medication of more than 0.5 mg/kg of methylprednisolone or its equivalent, evidence of uncontrolled infection, and refusal of managing physician to participate in the study.
On the day of study entry, we performed bronchoscopic bronchoalveolar lavage (BAL) for microbiologic examinations, procollagen peptide type III, and triggering receptor expressed on myeloid cells (TREM-1).
9 Methylprednisolone mixed in 200 mL of normal saline solution was administered continuously. A loading dose of 1 mg/kg was followed by an infusion of 1 mg/kg/day from day 1 to day 14, 0.5 mg/kg/day from day 15 to day 21, 0.25 mg/kg/day from day 22 to day 25, and 0.125 mg/kg/day from day 26 to day 28.
4 If patient was extubated between day 1 and 14, the patient was advanced to day 15 of treatment and tapered as scheduled. Lung protective ventilation strategy, including low tidal volume ventilation of less than 6 mL/kg of predicted body weight with permissive hypercapnia and fluid restriction, were applied during the study period. PEEP was adjusted based on airway pressure and was kept as high as possible without increasing the maximal inspiratory plateau pressure above 28 to 30 cmH
2O. Patients were assessed daily for weaning readiness and weaned by spontaneous breathing trial according to our protocol.
Data on demographic characteristics, physiologic characteristics, precipitating condition of ARDS, the Simplified Acute Physiology Score 3,
10 and Sequential Organ Failure Assessment
11 were recorded at study entry. The following baseline variables were also measured at enrollment: PF ratio, lung injury score (LIS), oxygenation index (OI), serum levels of C-reactive protein, procalcitonin, N-terminal pro-B-type natriuretic peptide (NT pro-BNP), interleukin-6 (IL-6), tumor necrosis factor-α, and transforming growth factor-β. At day 3 and 7, PF ratio, OI, LIS, and serum levels of C-reactive protein, procalcitonin, and NT pro-BNP were measured. Critical-illness-related corticosteroid insufficiency was evaluated at study entry by a delta serum cortisol level of <9 µg/dL after adrenocorticotropic hormone (250 µg) administration or random total serum cortisol <10 µg/dL.
12
The primary outcome was weaning from MV within 7 days or improvement in LIS of more than 1 point by study day 7.
4 For patients remaining intubated on study day 7, improvement in lung function was defined as follows: a reduction in LIS 1 or more point, and a day 7 LIS ≤2.0 (for study entry LIS ≤2.9) or ≤2.5 (for study entry LIS ≥3.0) as previously described.
4 Finally, we documented the outcomes of patients with ARDS including lengths of stays in ICU and hospital, and 28-day, ICU, and hospital mortality.
Data are presented as medians and interquartile range (IQR) for continuous variables and as numbers (percentages) for categorical variables. Data were compared using the Mann-Whitney U test for continuous variables and the Fisher's exact test for categorical variables. All tests were two-sided, and a p value <0.05 was considered significant. Data were analyzed using IBM SPSS Statistics 19.0 (IBM, Chicago, IL, USA).
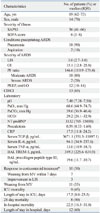
Over the study period, a total of 20 patients with ARDS who met inclusion criteria were enrolled into the study. Baseline characteristics are presented in
Table 1. The sample included 14 males and 6 females with a median age of 69 (62-73) years. The condition most often precipitating ARDS in the patient sample was pneumonia (90%). The median LIS and PF ratios were 3.0 (IQR, 2.7-3.0) and 146.6 (119.9-179.4), respectively. All but 2 patients were classified as moderate ARDS by the Berlin definition. The median levels of TREM-1 and procollagen peptide type III in BAL were 349.3 (225.6-634.9) pg/mL and 19.6 (11.7-39.7) pg/mL, respectively. After 7 days of corticosteroid treatment, 10 (50%) patients showed response to the treatment (successful extubation in 7 and 1-point reduction in LIS in 3).
Clinical characteristics and outcomes of responders and non-responders to prolonged corticosteroid treatment are presented in
Table 2. In comparisons of potential predictors between responders and non-responders, responders had higher initial PF ratios (170.5 vs. 127.2,
p=0.023) and lower levels of TREM-1 in BAL fluid (313.6 pg/mL vs. 520.5 pg/mL,
p=0.029) than non-responders. In addition, responders had greater reduction in LIS on day 3 (-1 vs. 0,
p<0.001) than non-responders. Hospital mortality was significantly lower in patients who responded to corticosteroid treatment (30%) than those who did not respond (90%) (
p=0.020).
In the present study, we evaluated early predictors of response to prolonged corticosteroid treatment in early ARDS. Greater reduction in LIS after 3 days of the treatment was associated with response to treatment, although higher PF ratios and lower level of TREM-1 in BAL fluid were also associated with response to treatment.
The use of corticosteroids for treating ARDS has been the subject of great controversy and debate. Trials of high-dose, short course corticosteroids for early-phase ARDS failed to show improvements in survival.
13,
14 However, a recent trial using low-dose, prolonged course of corticosteroids showed that corticosteroid-induced down-regulation of systemic inflammation was associated with significant improvements in pulmonary and extrapulmonary organ dysfunction.
4 In addition, changes of surrogate markers for pulmonary and systemic inflammation were observed within 3 days after treatment, consistent with previous studies of moderate-dose, prolonged corticosteroid treatment for late ARDS.
1,
15 These changes were associated with parallel improvements in pulmonary and extrapulmonary organ dysfunction scores.
1,
4 Therefore, response to prolonged corticosteroid treatment for ARDS in terms of changes of organ dysfunction scores are expected within 3 days after the initiation of treatment. In the present study, reduction of LIS, used for surrogate marker for pulmonary dysfunction, at day 3 after corticosteroid treatment was associated with response to the treatment by a day. Based on these findings, therefore, treatment response should be monitored by daily measurements of LIS when patients undergo prolonged corticosteroid treatment for ARDS.
Corticosteroids may induce serious adverse events including superinfection, hyperglycemia, and muscle weakness, all of which deserve specific attention for prevention. Such drug-related adverse events could discourage physicians from treating ARDS patients with corticosteroids. In addition, blunted febrile response in patients treated with corticosteroids is also important, since signs of uncontrolled infection could be masked. In the present study, the levels of TREM-1 in the BAL fluid of non-responders were higher than those of responders to corticosteroid treatment. The level of TREM-1 in BAL fluid has been suggested as a biomarker of bacterial pneumonia.
9 In patients with high levels of TREM-1 in BAL fluid, therefore, pulmonary infection, which is a precipitating condition of ARDS, might not be controlled prior to corticosteroid treatment. In these patients, corticosteroid treatment may lead to negative effects that counterbalance the positive effects of this agent.
The limitations of this study are attributed primarily to univariate analysis with a small sample size, with which were unable to evaluate the independent contributions of each predictor of response to corticosteroid treatment. Therefore, a larger scale study is needed to confirm the findings of this pilot study.
In summary, response to prolonged corticosteroid treatment in patients with early ARDS was observed in 50% of the patients in our sample. PF ratios and TREM-1 levels in BAL fluid at baseline, and reduction in LIS 3 days after treatment, were associated with response to prolonged low-dose corticosteroid treatment. However, this observation needs to be further evaluated by a multicenter, prospective study with larger sample size.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download