Abstract
Purpose
To investigate the molecular responses of various genes and proteins related to disc degeneration upon treatment with cytokines that affect disc-cell proliferation and phenotype in living human intervertebral discs (IVDs). Responsiveness to these cytokines according to the degree of disc degeneration was also evaluated.
Materials and Methods
The disc specimens were classified into two groups: group 1 (6 patients) showed mild degeneration of IVDs and group 2 (6 patients) exhibited severe degeneration of IVDs. Gene expression was analyzed after treatment with four cytokines: recombinant human bone morphogenic protein (rhBMP-2), transforming growth factor-β (TGF-β), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α). Molecular responses were assessed after exposure of cells from the IVD specimens to these cytokines via real-time polymerase chain reaction and immunofluorescence staining.
Results
mRNA gene expression was significantly greater for aggrecan, type I collagen, type II collagen, alkaline phosphatase, osteocalcin, and Sox9 in group 1 than mRNA gene expression in group 2, when the samples were not treated with cytokines. Analysis of mRNA levels for these molecules after morphogen treatment revealed significant increases in both groups, which were much higher in group 1 than in group 2. The average number of IVD cells that were immunofluorescence stained positive for alkaline phosphatase increased after treatment with rhBMP-2 and TGF-β in group 1.
Degeneration of the intervertebral disc (IVD) is a natural aging process. Healthy IVDs exhibit high water content in the nucleus pulposus (NP), as its matrix is rich in large aggregating proteoglycans.1,2 With degeneration, there is a progressive loss of the proteoglycan matrix, which normally functions to retain water. This causes dehydration and desiccation within the NP, and the IVD becomes a more fibrotic and less cartilaginous structure.3,4,5,6
Degeneration of IVDs is clinically associated with lower back pain and other important diseases of the spine. Treatment options range from pain management to invasive procedures, such as discectomy, intradiscal electrothermal therapy, fusion, and spinal arthroplasty. However, these options address the clinical symptoms of degeneration of the IVD rather than targeting the pathophysiological pathways involved in the degenerative process.
Genetic, mechanical, and biologic factors are widely regarded as important contributors to the degenerative process.7,8,9,10,11,12,13,14 To better characterize the degenerative process and to prevent or reverse degenerative changes in the disc matrix by altering disc matrix metabolism, many investigators have conducted biological experiments and attempted treatment of disc degeneration utilizing cellular components, matrix-derivatives, and molecules known to influence disc cell metabolism and phenotype.15,16,17,18,19 There are at least four different classes of molecules that are currently being investigated for disc therapy: anti-catabolics, mitogens, morphogens, and intracellular regulators. All of these molecules have some in vitro data supporting their use; however, few have been tested in animal models of disc degeneration to verify the biological mechanisms of each molecule. There is no clinically proven biological therapy for degeneration of human IVDs.7,9,10,11,15,16,19,20,21,22,23,24,25,26 The results of biological therapies may differ according to the therapeutic modalities used and the degree of degeneration, both of which are further related to the chemical composition and histologic changes of the IVD.25,27,28,29,30
The aging process changes the expression levels and spatial distribution of transforming growth factor (TGF) and bone morphogenic protein (BMP) molecules and receptors.12,31 Okuda, et al.13 supported this notion by demonstrating that the responsiveness of intervertebral cells to insulin-likegrowth factor-1 (IGF-1) and TGF-β decreases with advancing age in rabbit disc cells. Thompson, et al.32 were the first to demonstrate that exogenous administration of a growth factor, TGF-1, can significantly increase proteoglycan synthesis in NP cells in vitro. This prompted further investigation into other growth factors such as IGF-1, BMP-2, and BMP-7, all of which have been shown to enhance the anabolic functions of IVD cells by up-regulating proteoglycan synthesis.33,34 Degeneration of IVDs may reflect molecular biologic changes in the IVD with age, and the degree of IVD degeneration may be influence the responsiveness of IVDs to treatment with cytokines. Nevertheless, the biologic responsiveness living human IVDs to cytokine treatment in relation to degeneration grade has not been studied.
Accordingly, the objective of this study was to investigate the molecular responses of various genes and proteins related to disc degeneration upon treatment with cytokines known to influence disc-cell proliferation and phenotype in living human IVD cells obtained from patients undergoing discectomy. As well, responsiveness to these cytokines was assessed according to the degree of degeneration in living human IVD cells.
Living human disc specimens were obtained from 12 patients who underwent discectomy for degenerative lumbar disc herniation and who were unresponsive to conservative therapy. Exclusion criteria included infection, metabolic bone disease and neoplastic disease. MRI (Magneton Vision 1.5T, Siemens, Erlangen, Germany) was performed for all patients. The PACS software and PACS workstation (Centricity 2.0, General Electrics Medical Systems, Milwaukee, WI, USA) was used for review by an independent neurosurgeon and neuroradiologist. Disc degeneration was graded on routine T2-weighted MRI images using the Pfirrmann grading system. The disc specimens were classified into two groups: patients in group 1 (6 patients) exhibited mild degeneration of IVDs (Grade II and III), while patients in group 2 (6 patients) showed severe degeneration of IVDs (Grade IV and V). This study was approved by the Institutional Review Board of our institute (No. 6-2008-0290).
Unless otherwise stated, all reagents were purchased from GibcoBRL (Grand Island, NY, USA). Intervertebral disc materials were collected from patients during discectomy. To make the samples homologous, disc materials were acquired from the nucleus pulposus and not the annulus. Tissues from each disc were dissected into small pieces and incubated (5% CO2, 95% room air at 37℃) in Dulbecco's Modified Eagle Medium and Ham's F-12 (DMEM/F-12) media. To isolate the cells, disc tissue was digested in DMEM/F-12 media with 0.2% protease (Sigma Chemical, St. Louis, MO, USA) for 1 hour, followed by 0.025% collagenase (Sigma Chemical) for 12 hours. Cells from less than two passages were used for each experiment. Each sample of disc cells (2×105 cells/well) was grown as a monolayer culture for 6 days in DMEM/F-12 media with 10% fetal bovine serum (FBS), 10 U/mL penicillin, 10 g/mL streptomycin, and 0.2 mmol/L L-glutamine. After 6 days, mRNA expression of aggrecan, type I collagen, type II collagen, Sox9, alkaline phosphatase, osteocalcin, and glyceraldehydes phosphate dehydrogenase (GAPDH) was assayed using the complete mRNA sequence from the National Center for Biotechnology Information. Forward and reverse primer sequences of aggrecan, type I collagen, type II collagen, Sox9, osteocalcin, and alkaline phosphatase are listed in Table 1.
Samples from each experimental group were also cultured in a chamber slide in an incubator (5% CO2, 95% room air at 37℃) at 3×104 cells/chamber. When the cell culture became confluent, the media was replaced with DMEM/F-12 media containing 1% FBS+10 U/mL penicillin, 10 g/mL streptomycin, 0.2 mmol/l L-glutamine, and 5 µg/mL vitamin C. The gene expression after treatment with four cytokines was analyzed. Recombinant human bone morphogenic protein-2 (rhBMP-2) and TGF-β were used as morphogens for the disc cells. Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) were treated as inflammatory mediators implicated in disc degeneration. mRNA expression from disc cell culture without cytokines was used as a baseline control value. Disc cells were cultured in monolayer and treated with rhBMP-2 100 ng/mL (R&D System, Minneapolis, MN, USA), TGF-β 10 ng/mL (Invitrogen, Carlsbad, CA, USA), IL-1β 10 ng/mL (Invitrogen) and TNF-α 10 ng/mL (Invitrogen) for 6 days. On day 3, the culture media were changed with the same concentrations of rhBMP-2, TGF-β, IL-1β, and TNF-α in each well.
An ABI Prism 7300 (Applied Biosystemz, Foster City, CA, USA) was used to detect SYBR Green fluorescent dye incorporated in double-stranded DNA. A 20 µL reaction volume included 25 ng of cDNA from real-time polymerase chain reaction (RT-PCR) and 5 pmole of each primer (aggrecan, alkaline phosphatase, type I collagen, type II collagen, osteocalcin, and Sox9). Forty RT-PCR cycles were performed for denaturation (95℃ for 30 seconds), annealing, and elongation (60℃ for 60 seconds). To confirm amplification specificity, PCR products were subjected to a melting curve analysis. Threshold cycles (Ct) of aggrecan, alkaline phosphatase, type I collagen, type II collagen, osteocalcin, and Sox9 were standardized according to GAPDH. The mRNA expression of group 1 was compared to group 2 and reported as a ratio.
Each cell sample (3×104 cells/well) was grown as a monolayer culture in the DMEM/F-12 culture media containing 1% FBS, 10 U/mL penicillin, 10 g/mL streptomycin, 0.2 mmol/L L-glutamine, and 5 µg/mL vitamin C for 3 days in the incubator (5% CO2, 95% room air at 37℃). The cultured cells were fixed with 100% EtOH and then washed with 10 mM PBS solution (Sigma, St. Louis, MO, USA). Monoclonal anti-aggrecan, anti-alkaline phosphatase, anti-type I collagen, anti-type II collagen, anti-osteocalcin, and anti-Sox9 were applied at 4℃ overnight. After washing, secondary antibody conjugated with fluorescein isothiocyanate (FITC) was applied to the wells (room temperature, 2 hours). The immunoreactivity of the IVD cells for rhBMP-2 and TGF-β was analyzed to examine the chondrogenic activity. The wells were then rinsed, mounted and photographed with a fluorescence photomicroscope (NIKON microphot-SA, Kogaku, Japan). For immunofluorescence, fluoroch-romes on the sections were exited using a 510 nm emission filter for a green fluorescent protein, and a 580 nm emission filter for a secondary antibody with FITC. We counted the average number of immunofluorescence-positive-stained IVD cells for anti-aggrecan, anti-alkaline phosphatase, anti-type I collagen, anti-type II collagen, anti-osteocalcin, and anti-Sox9 in both group 1 and group 2. Five out of nine wells were scanned using light microscopy. The number of immunofluorescence-positive-stained IVD cells in each of the five wells was tabulated and extrapolated to the total of nine wells. The average number of immunofluorescence-positive-stained IVD cells was compared as the average number of cells without cytokine treatment and expressed as a ratio.
Using the Pfirrmann grading system, the discs of 12 patients were classified into two groups: group 1 (6 patients) contained mildly degenerated discs (grade II; 3 patients and grade III; 3 patients) and group 2 (6 patients) contained severely degenerated discs (grade IV; 2 patients and grade V; 4 patients). The mean patient age in group 2 (59.9±13.9 year) was significantly higher than group 1 (43.1±13.3 year) (p<0.05).
The mRNA levels of genes specific for aggrecan, type I collagen, type II collagen, alkaline phosphatase, osteocalcin, and Sox9 which were produced from forty RT-PCR cycles were analyzed.
mRNA gene expression in group 1 (mild degenerative IVD) was significantly greater for aggrecan, type I collagen, type II collagen, alkaline phosphatase, osteocalcin, and Sox9 than the mRNA gene expressions thereof in group 2 (severe degenerative IVD). The differences in mRNA gene expression between the two groups for aggrecan, type I collagen, type II collagen, alkaline phosphatase, and Sox9 were all statistically significant (Fig. 1).
In order to confirm the response to cytokines, the mRNA levels of these genes without cytokine treatment were used as a control. The values of each mRNA level of these genes were compared as a ratio with their mRNA levels without cytokine treatment.
The mRNA levels of aggrecan after treatment with morphogens increased by 5.46-fold with rhBMP-2 and 3.55-fold with TGF-β in group 1, and increased by 2.67-fold with rhBMP-2 and 2.30-fold with TGF-β in group 2. The mRNA expression of aggrecan after treatment with inflammatory mediators decreased by 0.48-fold with TNF-α and 0.61-fold with IL-1β in group 1 and decreased by 0.65-fold with TNF-α and 0.51-fold with IL-1β in group 2. The response to rhBMP-2 in group 1 was greater by 2.03-fold than that of group 2 (p<0.05). However, the response to others cytokines showed no statistical difference between the two groups (Fig. 2A).
The mRNA levels of alkaline phosphatase after treatment with morphogens increased by 4.82-fold with rhBMP-2 and 4.63-fold with TGF-β in group 1, and increased by 2.62-fold with rhBMP-2 and 2.31-fold with TGF-β in group 2. The mRNA expression of alkaline phosphatase after treatment with inflammatory mediators decreased by 0.59-fold with TNF-α and 0.54-fold with IL-1β in group 1, and decreased by 0.66-fold with TNF-α and 0.62-fold with IL-1β in group 2. The response to rhBMP-2 and TGF-β in group 1 was greater by 1.84 times and 2.00 times than those in group 2, respectively (p<0.05). The response to others cytokines demonstrated no statistical difference between the two groups (Fig. 2B).
The mRNA levels of type I collagen after treatment with morphogens increased by 4.35-fold with rhBMP-2 and 3.87-fold with TGF-β in group 1, and increased by 1.84-fold with rhBMP-2 and 2.54-fold with TGF-β in group 2. The mRNA expression for type I collagen after treatment with inflammatory mediators decreased by 0.84-fold with TNF-α and 0.75-fold with IL-1β in group 1, and decreased by 0.70-fold with TNF-α and 0.77-fold with IL-1β in group 2. The response to all cytokines showed no statistical difference between the two groups (Fig. 2C).
The mRNA levels of type II collagen after treatment with morphogens increased by 3.92-fold with rhBMP-2 and 3.59-fold with TGF-β in group 1, and increased by 2.51-fold with rhBMP-2 and 2.93-fold with TGF-β in group 2. The mRNA expression for type II collagen after treatment with inflammatory mediators decreased by 0.56-fold with TNF-α and 0.49-fold with IL-1β in group 1 and decreased by 0.67-fold with TNF-α and 0.67-fold with IL-1β in group 2. Response to rhBMP-2 in group 1 was 1.55 times greater than that of group 2 (p<0.05). However, the response to others cytokines showed no statistical difference between the two groups (Fig. 2D).
The mRNA levels of osteocalcin after treatment with morphogens increased by 4.83-fold with rhBMP-2 and 3.04-fold with TGF-β in group 1, and increased by 2.46-fold with rhBMP-2 and 1.77-fold with TGF-β in group 2. The mRNA expression for osteocalcin after treatment with inflammatory mediators decreased by 0.52-fold with TNF-α and 0.65-fold with IL-1β in group 1, and decreased by 0.76-fold with TNF-α and 0.67-fold with IL-1β in group 2. The response to TGF-β in group 1 was 1.72 times greater than that of group 2 (p<0.05). The response to others cytokines showed no statistical difference between the two groups (Fig. 2E).
The mRNA levels of Sox9 after treatment with morphogens increased by 5.17-fold with rhBMP-2 and 3.69-fold with TGF-β in group 1 and increased by 2.44-fold with rhBMP-2 and 2.41-fold with TGF-β in group 2. The mRNA expression for Sox9 after treatment with inflammatory mediators decreased by 0.53-fold with TNF-α and 0.68-fold with IL-1β in group 1 and decreased by 0.64-fold with TNF-α and 0.68-fold with IL-1β in group 2. The response to TGF-β in group 1 was 1.72 times greater than that in group 2 (p<0.05). The response to others cytokines showed no statistical difference between the two groups (Fig. 2F).
The average numbers of immunofluorescence-positive-stained IVD cells for aggrecan according to the control group (no treatment), rhBMP-2 100 ng/mL group and TGF-β 10 ng/mL group, were 6.20×103, 2.61×104, and 2.31×104, respectively, in group 1, and 5.04×103, 1.08×104, and 9.76×103, respectively, in group 2. The average numbers of immunofluorescence-positive-stained IVD cells for aggrecan increased by 4.21 times with rhBMP-2 and 3.72 times with TGF-β in group 1, and increased by 2.14 times with rhBMP-2 and 1.94 times with TGF-β in group 2. The responses to rhBMP-2 and TGF-β in group 1 were 1.96 times and 1.92 times greater than those in group 2, respectively (p< 0.05) (Table 2, Figs. 3 and 4).
For the control group (no treatment), rhBMP-2 100 ng/mL group, and TGF-β 10 ng/mL group, the average numbers of immunofluorescence-positive-stained IVD cells for alkaline phosphatase were 6.57×103, 2.45×104, and 2.41×104, respectively, in group 1 and 4.86×103, 1.22×104, and 1.12×104, respectively, in group 2. The average number of immunofluorescence-positive-stained IVD cells for alkaline phosphatase increased 3.73 times with rhBMP-2 and 3.66 times with TGF-β in group 1, and increased 2.52 times with rhBMP-2 and 2.29 times with TGF-β in group 2. The responses to rhBMP-2 and TGF-β in group 1 were 1.48 times and 1.59 times greater than those in group 2, respectively (p<0.05).
For the control group (no treatment), rhBMP-2 treatment group, and TGF-β treatment group, the average numbers of immunofluorescence-positive-stained IVD cells for type I collagen were 6.50×103, 2.67×104, and 2.56×104, respectively, in group 1, and 4.32×103, 7.92×103, and 8.69×103, respectively, in group 2. The average number of immunofluorescence-positive-stained IVD cells for type I collagen increased 4.11 times with rhBMP-2 and 2.01 times with TGF-β in group 1, and increased 1.83 times with rhBMP-2 and 1.95 times with TGF-β in group 2. The response to rhBMP-2 and TGF-β in group 1 were 2.24 times and 1.96 times greater than those in group 2, respectively (p<0.05).
For the same three treatment groups above, the average numbers of immunofluorescence-positive-stained IVD cells for type II collagen were 6.98×103, 2.53×104, and 2.42×104, respectively, in group 1 and 6.21×103, 1.47×104, and 1.59×104, respectively, in group 2. The average number of immunofluorescence-positive-stained IVD cells for type II collagen increased 3.62 times with rhBMP-2 and 3.47 times with TGF-β in group 1, and increased 2.38 times with rhBMP-2 and 2.56 times with TGF-β in group 2. The responses to rhBMP-2 and TGF-β in group 1 were 1.53 times and 1.35 times greater than those in group 2, respectively (p<0.05).
The average numbers of immunofluorescence-positive-stained IVD cells for osteocalcin in the three treatment groups above were 6.30×103, 2.23×104, and 2.08×104, respectively, in group 1, and 5.58×103, 1.37×104, and 1.10×104, respectively, in group 2. The average number of immunofluorescence-positive-stained IVD cells for osteocalcin increased 3.54 times with rhBMP-2 and 3.30 times with TGF-β in group 1, and increased 2.38 times with rhBMP-2 and 1.97 times with TGF-β in group 2. The responses to rhBMP-2 and TGF-β in group 1 were 1.49 times and 1.68 times greater than those in group 2, respectively (p<0.05).
For the control group (no treatment), rhBMP-2 treatment group, and TGF-β treatment group, the average numbers of immunofluorescence-positive-stained IVD cells for Sox9 were 6.60×103, 2.77×104, and 2.54×104, respectively, in group 1 and 5.76×103, 1.29×104, and 1.19×104, respectively, in group 2. The average number of immunofluorescence-positive-stained IVD cells for Sox9 increased 4.19 times with rhBMP-2 and 3.85 times with TGF-β in group 1, and increased 2.25 times with rhBMP-2 and 2.06 times with TGF-β in group 2. The responses to rhBMP-2 and TGF-β in group 1 were 1.86 times and 1.87 times greater than those in group 2, respectively (p<0.05).
Disc degenerative changes may be associated with pain, and treatment options for disc degeneration are limited. Among the various genes associated with matrix synthesis, type I and type II collagen act as fibrillar molecules; aggrecan is a core protein for sulfated glycosaminoglycans, Sox9 upregulates both aggrecan and type II collagen; and osteocalcin and alkaline phosphatase are markers of osteogenicity.35,36,37,38,39 Proinflammatory cytokines, such as TNF-α and IL, are well known to be associated with disc degradation.5,40,41,42
With degeneration, IVDs show down regulation of various genes for aggrecan, type II collagen, Sox9, type I collagen, alkaline phosphatase, osteocalcin and others.5,38,40 Various inflammatory mediators have also been implicated in the degeneration of the IVD and discogenic pain, including nitric oxide, ILs, matrix metalloproteinases, prostaglandin E2 and a group of cytokines.25 TNF-α and IL-1β are well known to be associated with disc degradation and discogenic back pain.26,27,43 TNF-α is an important initiator of matrix degeneration, whereas IL-1β plays a greater role in pathological degradation.
Many growth factors, including IGF-1, TGF-1, BMP-2, and BMP-7 have been extensively investigated by the cartilage research community, and have been shown to positively influence the metabolism and healing potential of cartilage.18,44,45,46 With this success and the similar chondrocytic composition of IVDs, investigations into the effects of growth factors on IVD cells have been performed by researchers. All of these molecules have some in vitro data regarding their effects, although few have been tested in vivo in animal models of disc degeneration to verify the biological mechanisms of each molecule. There is no clinically proven biological therapy for degeneration of human IVDs.
rhBMP-2, TGF-β, TNF-α, and IL-1β were used as morphogenic cytokines and inflammatory mediators in this study. The comparative response to these four cytokines between group 1 (mild degenerative IVD) and group 2 (severe degenerative IVD) were assayed in terms of gene and protein expression and a statistically significant difference was found. mRNA expression in mild degenerative IVD was significantly greater than that in severe degenerative IVDs for aggrecan, type I collagen, type II collagen, alkaline phosphatase, osteocalcin, and Sox9 before cytokine treatment. After the treatment with morphogens, mRNA levels for these molecules increased significantly in both groups, the effects were much greater in group 1. There was no significant difference between the groups after treatment with inflammatory cytokines, however. Micrographic analysis of rhBMP-2 and TGF-β immunoreactive IVD cells for aggrecan, alkaline phosphatase, type I collagen, type II collagen, osteocalcin, and Sox9 revealed similar results in both groups. Differences in cell proliferation upon treatment with rhBMP-2, TGF-β, TNF-α, and IL-1β in degenerative living human IVDs may differ according to type of cytokine and inflammatory mediator.
The average number of immunofluorescence-positive-stained IVD cells for alkaline phosphatase increased after treatment with rhBMP-2 and TGF-β in group 1. Microscopically, IVD cells showed strong immunoreactivity and well-demarcated configuration. Our results highlights several features: treatment with rhBMP-2 and TGF-β increases the expression of the various genes associated with matrix synthesis, including aggrecan, alkaline phosphatase, type I collagen, type II collagen, osteocalcin, and Sox9. Treatment with TNF-α and IL-1β decreases the expression of these genes. As separate treatment with rhBMP-2 or TGF-β showed significant increases in mRNA expression and cell proliferation, a synergic effect may be expected after treatment with rhBMP-2 combined with TGF-β. The results of the mRNA expression and immunofluorescence study revealed that rhBMP-2 and TGF-β play a role in cell metabolism and proliferation.
The molecular responses to treatment with rhBMP-2, TGF-β, TNF-α, and IL-1β in degenerative living human IVDs may differ according to the degree of IVD degeneration. Even though IVD cells from mild degenerative IVD specimens in group 1 showed greater molecular response to treatment with cytokines than those from severe degenerative IVD specimens in group 2 in this in vitro study, several factors should be considered when classifying IVD cells by degree of degeneration in living humans such as IVD cell count, the degree of hydration, elasticity, the existence of a vacuum disc, and intradiscal pressure. Therefore, the molecular responses to treatment with rhBMP-2, TGF-β, TNF-α, and IL-1β in degenerative living human IVDs may differ in an in vivo study.
Degeneration of the IVD is a complex process that disrupts an otherwise well-defined organization and biochemical balance. Many different biological treatment modalities have been studied to treat degenerative disc disease. Some bioactive molecules have been investigated for clinical applications. However, most of the data for the biologic responsiveness of various genes and proteins related to disc degeneration upon treatment with cytokines that influence disc-cell metabolism and phenotype has been obtained using animal IVDs. However, experimental data for animal IVDs cannot be applied to clinical treatment. In the present study, treatment with rhBMP-2 and TGF-β modulated molecular responses in degenerative living human IVD cells, and the molecular responses differed according to the degree of IVD degeneration. Thus, the degree of IVD degeneration may be an important factor to the responsiveness of IVD cells to biologic therapies in clinical application. Nevertheless, while cytokine treatment may be effective in treating both severe degenerative IVD and mild degenerative IVD, the potential clinical use of rhBMP-2 and TGF-β for treatment of degenerative IVD is limited due to their short biologic half-life. Chronic conditions like internal disc disruption (IDD) may require more prolonged and sustained cytokine levels in order to generate a therapeutic effect. This has led investigators to contemplate the potential use of gene therapy as a treatment modality.
Figures and Tables
Fig. 1
mRNA gene expression in group 1 (mild degenerative IVD) was significantly greater for aggrecan, type I collagen, type II collagen, alkaline phosphatase, osteocalcin, and Sox9 than mRNA gene expression in group 2 (severe degenerative IVD). *p<0.01. **p<0.05. IVD, intervertebral disc.

Fig. 2
The mRNA levels of aggrecan (A), alkaline phosphatase (B), type I collagen (C), type II collagen (D), osteocalcin (E), and Sox9 (F) after treatment with morphogens. vs. no treatment; *p<0.01 and **p<0.05. Group 1 vs. group 2; ‡p<0.01 and †p<0.05. TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; rhBMP-2, recombinant human bone morphogenic protein-2.

Fig. 3
Immunostaining of human IVD cells for aggrecan, alkaline phosphatase, type I collagen, type II collagen, osteocalcin, and Sox9 in group 1 (mild degenerative IVD) after rhBMP-2 and TGF-β treatment. IVD, intervertebral disc; rhBMP-2, recombinant human bone morphogenic protein-2; TGF-β, transforming growth factor-β.
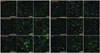
Fig. 4
Immunostaining of human IVD cells for aggrecan, alkaline phosphatase, type I collagen, type II collagen, osteocalcin, and Sox9 in group 2 (mild degenerative IVD) after rhBMP-2 and TGF-β treatment. IVD, intervertebral disc; rhBMP-2, recombinant human bone morphogenic protein-2; TGF-β, transforming growth factor-β.
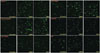
Table 1
Primer-Sequence for Real-Time PCR
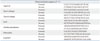
Table 2
Production Ratio of Immunofluorescence-Positive-Stained IVD Cells for rhBMP-2 and TGF-β

ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2008 (6-2008-0290).
References
1. Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine (Phila Pa 1976). 1987; 12:264–268.

2. Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976). 1995; 20:1307–1314.

3. Shvartzman L, Weingarten E, Sherry H, Levin S, Persaud A. Cost-effectiveness analysis of extended conservative therapy versus surgical intervention in the management of herniated lumbar intervertebral disc. Spine (Phila Pa 1976). 1992; 17:176–182.

4. Antoniou J, Demers CN, Beaudoin G, Goswami T, Mwale F, Aebi M, et al. Apparent diffusion coefficient of intervertebral discs related to matrix composition and integrity. Magn Reson Imaging. 2004; 22:963–972.

5. Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996; 98:996–1003.

6. Pritzker KP. Aging and degeneration in the lumbar intervertebral disc. Orthop Clin North Am. 1977; 8:66–77.

7. Takegami K, Thonar EJ, An HS, Kamada H, Masuda K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine (Phila Pa 1976). 2002; 27:1318–1325.

8. An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine (Phila Pa 1976). 2005; 30:25–31.

9. Takegami K, An HS, Kumano F, Chiba K, Thonar EJ, Singh K, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005; 5:231–238.

10. Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003; 24:3531–3541.

11. Watanabe K, Mochida J, Nomura T, Okuma M, Sakabe K, Seiki K. Effect of reinsertion of activated nucleus pulposus on disc degeneration: an experimental study on various types of collagen in degenerative discs. Connect Tissue Res. 2003; 44:104–108.

12. Matsunaga S, Nagano S, Onishi T, Morimoto N, Suzuki S, Komiya S. Age-related changes in expression of transforming growth factor-beta and receptors in cells of intervertebral discs. J Neurosurg. 2003; 98:1 Suppl. 63–67.

13. Okuda S, Myoui A, Ariga K, Nakase T, Yonenobu K, Yoshikawa H. Mechanisms of age-related decline in insulin-like growth factor-I dependent proteoglycan synthesis in rat intervertebral disc cells. Spine (Phila Pa 1976). 2001; 26:2421–2426.

14. Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, et al. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996; 14:690–699.

15. Gruber HE, Hanley EN Jr. Biologic strategies for the therapy of intervertebral disc degeneration. Expert Opin Biol Ther. 2003; 3:1209–1214.

16. Brisby H, Tao H, Ma DD, Diwan AD. Cell therapy for disc degeneration--potentials and pitfalls. Orthop Clin North Am. 2004; 35:85–93.

17. Handa T, Ishihara H, Ohshima H, Osada R, Tsuji H, Obata K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine (Phila Pa 1976). 1997; 22:1085–1091.

18. Martinek V, Ueblacker P, Imhoff AB. Current concepts of gene therapy and cartilage repair. J Bone Joint Surg Br. 2003; 85:782–788.

19. Nishida K, Doita M, Takada T, Shimomura T, Maeno K, Kakutani K, et al. [Biological approach for treatment of degenerative disc diseases]. Clin Calcium. 2005; 15:79–86.
20. Alini M, Roughley PJ, Antoniou J, Stoll T, Aebi M. A biological approach to treating disc degeneration: not for today, but maybe for tomorrow. Eur Spine J. 2002; 11:Suppl 2. S215–S220.

21. Sato M, Asazuma T, Ishihara M, Ishihara M, Kikuchi T, Kikuchi M, et al. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine (Phila Pa 1976). 2003; 28:548–553.

22. Luk KD, Ruan DK, Chow DH, Leong JC. Intervertebral disc autografting in a bipedal animal model. Clin Orthop Relat Res. 1997; 13–26.

23. Pfeiffer M, Boudriot U, Pfeiffer D, Ishaque N, Goetz W, Wilke A. Intradiscal application of hyaluronic acid in the non-human primate lumbar spine: radiological results. Eur Spine J. 2003; 12:76–83.

24. Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976). 2000; 25:3005–3013.
25. Guiot BH, Fessler RG. Molecular biology of degenerative disc disease. Neurosurgery. 2000; 47:1034–1040.

26. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford). 2008; 47:809–814.

27. Lipson SJ, Muir H. 1980 Volvo award in basic science Proteoglycans in experimental intervertebral disc degeneration. Spine (Phila Pa 1976). 1981; 6:194–210.
28. Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987; 5:198–205.

29. Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976). 2002; 27:2631–2644.
30. Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976). 2004; 29:2691–2699.
31. Oegema TR Jr. The role of disc cell heterogeneity in determining disc biochemistry: a speculation. Biochem Soc Trans. 2002; 30(Pt 6):839–844.

32. Thompson JP, Oegema TR Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine (Phila Pa 1976). 1991; 16:253–260.

33. Kim DJ, Moon SH, Kim H, Kwon UH, Park MS, Han KJ, et al. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine (Phila Pa 1976). 2003; 28:2679–2684.

34. Park JS, Nagata K. [BMP and LMP-1 for intervertebral disc regeneration]. Clin Calcium. 2004; 14:76–78.
35. Kuh SU, Zhu Y, Li J, Tsai KJ, Fei Q, Hutton WC, et al. A comparison of three cell types as potential candidates for intervertebral disc therapy: annulus fibrosus cells, chondrocytes, and bone marrow derived cells. Joint Bone Spine. 2009; 76:70–74.

36. Diefenderfer DL, Brighton CT. Microvascular pericytes express aggrecan message which is regulated by BMP-2. Biochem Biophys Res Commun. 2000; 269:172–178.

37. Fassett DR, Kurd MF, Vaccaro AR. Biologic solutions for degenerative disk disease. J Spinal Disord Tech. 2009; 22:297–308.

38. Adam M, Deyl Z. Degenerated annulus fibrosus of the intervertebral disc contains collagen type II. Ann Rheum Dis. 1984; 43:258–263.

39. Kim KS, Yoon ST, Park JS, Li J, Park MS, Hutton WC. Inhibition of proteoglycan and type II collagen synthesis of disc nucleus cells by nicotine. J Neurosurg. 2003; 99:3 Suppl. 291–297.

40. Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007; 6:247–261.

41. Séguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976). 2005; 30:1940–1948.

42. Rutges JP, Kummer JA, Oner FC, Verbout AJ, Castelein RJ, Roestenburg HJ, et al. Increased MMP-2 activity during intervertebral disc degeneration is correlated to MMP-14 levels. J Pathol. 2008; 214:523–530.

43. Yoon ST, Patel NM. Molecular therapy of the intervertebral disc. Eur Spine J. 2006; 15:Suppl 3. S379–S388.

44. Kuh SU, Zhu Y, Li J, Tsai KJ, Fei Q, Hutton WC, et al. Can TGF-beta1 and rhBMP-2 act in synergy to transform bone marrow stem cells to discogenic-type cells? Acta Neurochir (Wien). 2008; 150:1073–1079.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download