Abstract
Purpose
To analyze treatment outcome and side effects of adjuvant radiotherapy using radiotherapy fields and doses which have evolved over the last two decades in a single institution.
Materials and Methods
Forty-one patients received radiotherapy after orchiectomy from 1996 to 2007. At our institution, the treatment field for stage I seminoma has changed from dog-leg (DL) field prior to 2003 to paraaortic (PA) field after 2003. Fifteen patients were treated with the classic fractionation scheme of 25.5 Gy at 1.5 Gy per fraction. Other patients had been treated with modified schedules of 25.05 Gy at 1.67 Gy per fraction (n=15) and 25.2 Gy at 1.8 Gy per fraction (n=11).
Results
With a median follow-up of 112 months, the 5-year and 10-year survival rates were 100% and 96%, respectively, and 5-year and 10-year relapse-free survival rates were both 97.1%. No in-field recurrence occurred. Contralateral seminoma occurred in one patient 5 years after treatment. No grade III-IV acute toxicity occurred. An increased rate of grade 1-2 acute hematologic toxicity was found in patients with longer overall treatment times due to 1.5 Gy per fraction. The rate of grade 2 acute gastrointestinal toxicity was significantly higher with DL field than with PA field and also higher in the 1.8-Gy group than in the 1.5-Gy and 1.67-Gy groups.
Testicular cancer is the most frequent germ cell tumor in men aged 15-35 years. Its prevalence has been increasing annually up to 2% worldwide over the past 40 years,1,2 and seminoma accounts for approximately one third of testicular cancer cases.3 In the treatment of early seminoma, adjuvant radiotherapy (RT), active surveillance, or chemotherapy may be selected as treatment options after radical orchiectomy. Irrespective of treatment modality, the 5-year survival rate and disease-specific survival rate are more than 97% and 99-100%, respectively, indicating a very high cure rate.4
Postoperative adjuvant radiotherapy has long been considered as a standard treatment option for early testicular seminoma. However, alternative treatment options, such as surveillance and single-drug chemotherapy, have recently been conducted to replace adjuvant radiotherapy.5,6 Researchers who support surveillance without postoperative adjuvant therapy claim that no significant difference in overall survival (OS) is found between surveillance and adjuvant therapy, given that the rate of successful salvage treatment via radiotherapy or chemotherapy is high in case of recurrence. However, compared to the recurrence rate of 3-5% after adjuvant radiotherapy, surveillance resulted in a higher recurrence rate of 15-20%, which may increase economic burden and anxiety among patients.7 Postoperative carboplatin chemotherapy was reported to have treatment efficacy comparable to that of radiotherapy.6 However, only short-term follow-up data is available for carboplatin-based chemotherapy, and one must justify administering systemic treatment for a disease of which most common pattern of failure is local recurrence.
Meanwhile, acute and chronic complications caused by radiotherapy have been significantly reduced over the past 20 years by reducing radiation field and total dose while main-taining a high cure rate. The radiation field has changed from treating the lymph nodes both in the mediastinum and below diaphragm to treating only the lymph nodes below the diaphragm. More recently, the radiation field has been further reduced from the traditional dog-leg (DL) field, which includes the ipsilateral pelvic lymph nodes and aortic lymph nodes, to the paraaortic (PA) field, which includes the aortic lymph nodes only.8 Radiation dose has also been gradually reduced from 40-50 Gy to 20-25 Gy.9
This study was conducted to investigate the outcomes of adjuvant radiotherapy for stage I testicular seminoma and to analyze the impact of evolution of radiotherapy field and fractionation schemes over the last two decades on treatment-related toxicity.
This study was retrospectively conducted on 41 patients who underwent radiotherapy after orchiectomy for stage I testicular seminoma from 1996 to 2007. The study was approved by the Institutional Review Board of our institution (4-2012-0821). Those who had a previous history of other malignant tumors or who had previous radiotherapy or chemotherapy before being diagnosed with testicular seminoma were excluded from this study. At the time of initial diagnosis, all patients underwent physical examination, hematologic testing for tumor markers, such as alpha-fetoprotein (AFP), beta-human choriogonadotropin (β-HCG), and lactate dehydrogenase (LDH), chest X-ray, ultrasonography, and abdominal pelvic CT.
The median age of the patients was 34 years (range 21-56 years). The main symptoms observed during the diagnosis included scrotal masses and swelling. Among the 41 patients, five patients (12%) had pain in addition to scrotal masses and swelling. Right testicular involvement was more frequently found. Eleven patients (27%) had a history of cryptorchidism. Increased LDH and beta-HCG were observed in approximately one third of the patients, but increased AFP was found in none of the patients.
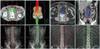
Radiotherapy was conducted using a linear accelerator, with 10-MV photon delivered in anteroposterior and posteroanterior apposing fields. At our institution, the DL field (Fig. 1A) had been used prior to 2003, while the PA field (Fig. 1B) has been mainly used in more recent years. In the current study, 26 patients were treated with DL field and 15 patients with PA field (Table 1). The margin of the radiation field for the PA field was defined as the superior border of the T11 vertebrate and the inferior border of the L5 vertebrate. For the DL field, the inferior border was the top of the obturator foramen, and the lateral field included the tips of the transverse process of the lumbar vertebra. For left-sided tumors, the lateral field was widened to include the left renal hilar nodes. If patients had a left testicular tumor, their left renal hilum was also included. A clamshell was used for the protection of the opposite testis upon treatment. Radiotherapy with three fractionation schedules was conducted according to the physician's preference: 15 patients received total 25.5 Gy at a daily dose of 1.5 Gy per fraction, 15 patients received total 25.05 Gy at 1.67 Gy per fraction, and 11 patients received total 25.2 Gy at 1.8 Gy per fraction. The median treatment period of radiotherapy was 23 days (range 21-27 days) for fractional doses of 1.5 Gy, 19 days (range 17-23 days) for 1.67 Gy, and 19 days (range 13-22 days) for 1.8 Gy.
Patients were followed every 6 months for two years after radiotherapy and every year thereafter. The patients were examined clinically at each follow-up, blood tests and a chest X-ray were obtained, and abdominal pelvic CT was conducted. Toxicity was evaluated using the Radiation Therapy Oncology Group toxicity criteria. Treatment-related adverse events occurring 6 months after completion of treatment were considered as late toxicity. The relationship of radiation field and dose with adverse events was examined using Spearman's rho. The survival period of the patients was obtained using the Kaplan-Meier method. Significance was defined as p-values of less than 0.05.
The median follow-up period was 112 months (range 50-200 months). The 5-year and 10-year OS rates of the total patients were 100% and 96.0%, respectively (Fig. 2A). During the follow-up period, one patient died of intercurrent disease 83 months after radiotherapy. The 5-year and 10-year relapse-free survival (RFS) rates of the total patients were both 97.1% (Fig. 2B). No recurrence was found within the radiation field. However, seminoma was found in the opposite testis in one patient 57 months after radiotherapy; the patient underwent salvage orchiectomy and was reported after 10 years to be free of disease.
During radiotherapy and the follow-up period, no grade 3-4 acute toxicity was observed. Grade 1-2 hematologic toxicity occurred in 22 patients (54%); of these patients grade 1 and grade 2 leukopenia occurred in 14 patients (34%) and 8 patients (20%). Only one patient who had grade 1 leukopenia experienced thrombocytopenia, and no patient experienced anemia. As for gastrointestinal (GI) toxicity, grade 1-2 nausea occurred, but no other symptom was found.
Radiotherapy-associated acute toxicity was further analyzed according to radiation field and fractionation scheme (Table 2). The frequency of grade 1-2 leukopenia and nausea requiring anti-emetics (grade 2) was set as a determinant for radiotherapy toxicity. In the analysis of the correlation of radiation field with hematologic toxicity using Spearman's rho, the increase in the rate of grade 1-2 hematologic toxicity was more significant in the DL field group (65%) than in the PA field group (33%) (p=0.049). As for the fractionation scheme, increased toxicity was significant in the 1.5-Gy group (87%), whose overall treatment time for radiotherapy was approximately one week longer, compared with 1.67-Gy (33%) and 1.8-Gy (36%) groups (grade 1-2 toxicity, p=0.010). When a partial correlation was examined by excluding the interaction of these two factors, a statistical significance was only found for fractionation scheme (p=0.007). When the radiation field and fractionation schemes were compared with the incidence of upper GI toxicity requiring anti-emetics using Spearman's rho, no significant difference in toxicity was found between the PA field group and the DL field group (p=0.161), but the toxicity significantly increased in the 1.8-Gy group, to the extent that anti-emetics were required in all the patients treated with 1.8 Gy per fraction (p=0.002). Interestingly, when a partial correlation was examined by excluding the interaction between RT field and fractionation scheme, a statistical significance was found for both RT field (p=0.035) and fractionation scheme (p=0.001). In summary, an increase in hematologic toxicity depended more on the fractionation scheme, particularly in the case of the 1.5-Gy group, than on the RT field. As for GI disorders requiring anti-emetics, toxicity was affected by both RT field and fractionation scheme, increasing more in the DL field group than in the PA field group and more in the 1.8-Gy group than in the 1.5-Gy and 1.67-Gy groups. Thus, among the three fractionation schemes, 1.67 Gy per fraction showed the best toxicity profile for both hematologic toxicity and GI disorders.
No chronic complication that was directly associated with radiotherapy occurred during the follow-up period; however, acute myeloid leukemia (AML; M3) occurred in one patient 80 months after radiotherapy.
At our institution, the results of adjuvant radiotherapy after surgical treatment for early testicular seminoma were excellent: the 10-year OS rate was 96.0%, and the RFS rate was 97.1%, with no recurrence except for seminoma that occurred in the contralateral testis. Potential causes of contralateral testicular seminoma consist of de novo metachronous primary malignancy, recurrence due to the metastasis of the primary lesion, and secondary malignancy due to radiation scattering. In a multi-institutional study conducted by Kamba, et al.,10 the recurrence rate of contralateral testis was similar between the surveillance (21.1%) and RT (22.2%) groups. Considering the aforementioned result, it is likely that contralateral testicular seminoma is attributable to de novo metachronous primary malignancy.
Rationale for active surveillance or single-agent chemotherapy after orchiectomy is the assumption that radiotherapy causes significant toxicity, in particular, increased rate of second malignancy, which may be attributed to the results of previous studies involving large-field and higher-dose radiotherapy. Modern radiotherapy schemes do not cause the adverse events observed in the previous treatments and minimize treatment-associated toxicity by significantly reducing radiation dose and treatment field.11 Thus, the rationale for the alternative treatment regimens should be re-evaluated based on the treatment outcomes and adverse events of modern radiotherapy.
The occurrence of secondary malignancy is an important issue among treatment-related chronic complications. In the current study, AML occurred only in one patient 80 months after radiotherapy. Due to the limited statistical power, it was difficult to confirm any direct correlation of AML with the radiotherapy. However, as secondary malignancies may occur due to genetic predisposition, immunodeficiency, common carcinogenic influences, and other environmental factors, it is important to accurately investigate the interaction of these factors with radiotherapy.12 For example, in the early era of Hodgkin's lymphoma treatment, radiation was delivered to a large field including entire lymph nodes in both sides of the diaphragm, at a dose of up to 40-50 Gy. However, in the current treatment of Hodgkin's lymphoma, the treatment field is gradually reduced to involved-field or involved-nodal areas, and radiation is delivered at a decreased dose of 20-30 Gy.13 A recent study reported that radiation treatment for Hodgkin's lymphoma with reduced field size and radiation dose resulted in no additional risk of secondary malignancy. In a comparative study that was conducted on the largest number of patients with Hodgkin's lymphoma, Koshy, et al.14 reported that no additional secondary malignancy was observed among patients who underwent a combination treatment of chemotherapy and radiotherapy in comparison with patients who were treated with chemotherapy alone. This finding suggests that radiotherapy does not cause secondary malignancy except for those that occur due to chemotherapy for the treatment of Hodgkin's lymphoma. In a similar manner, accurate evaluation of adverse events is required for the recently standardized treatment of seminoma, such as infradiaphragmatic and PA field irradiation and radiation doses of less than 30 Gy.
Alternative treatment modalities also have limitations. At our institution, 13 patients were followed who had received orchiectomy between 1996 and 2007 without receiving adjuvant radiotherapy: 4 patients after chemotherapy and 9 patients without chemotherapy. The median follow up for these patients was 62 months (range 19-138 months). One patient who was followed without adjuvant treatment experienced regional recurrence 18 months after orchiectomy and was 33-month disease-free after salvage chemotherapy at the time of writing. Among the 4 patients who were treated with chemotherapy, grade 3 leukopenia was observed in 2 patients and grade 1 leukopenia in 1 patient; however, toxicity could not be evaluated in a patient who had received chemotherapy overseas. A high recurrence rate of 15-20% after active surveillance is alarming despite the expectation that high cure rates can be achieved with salvage treatment.7 In addition, the late-relapsing characteristic of testicular seminoma contributes to several disadvantages of active surveillance such as increased economic burden attributable to long-term follow-ups via annual CT examination, concerns about radiation exposure due to multiple CT scans in young patients, and long-term anxiety in both patient and doctor. In the case of chemotherapy, the follow-up period is still insufficient to analyze the rate of late recurrence and the occurrence of chemotherapy-induced secondary malignancy.6 The result of a recent study showed that testicular intraepithelial neoplasia still persisted after administration of chemotherapy in patients who were diagnosed with testicular intraepithelial neoplasia in a testicular biopsy.15 Chemotherapy seems to delay the occurrence of contralateral testicular seminoma rather than preventing it.16 Considering the possibility that contralateral testicular seminoma may occur as a de novo metachronous primary malignancy, resulting in a high salvage rate via orchiectomy, systemic chemotherapy may not be a suitable option for the prevention of contralateral testicular seminoma, which has a recurrence rate of only 2%.
The current study is meaningful in that it was conducted on the patients who underwent standardized radiotherapy with a long term follow-up at a single institution, and that acute and chronic adverse events were analyzed in detail in relation to radiation field and dose. As for acute toxicity, many studies reported that no severe adverse events except for mild toxicity occurred after modern radiotherapy.9,17 Among studies that have been conducted to determine the minimum radiation field and total dose that can effectively reduce toxicity in the radiotherapy of testicular seminoma, Medical Research Council TE18 and European Organization for Research and Treatment of Cancer Trial 30942 showed that a total dose of 20 Gy irradiated to patients for two weeks resulted in the same treatment outcomes, mild acute toxicity, and no late complications.9 In the current study, most of the patients underwent radiotherapy at a consistent dose of 25 Gy, and no acute toxicity related to radiotherapy occurred except for grade 1-2 leukopenia and nausea. Furthermore, no chronic complications directly asso-ciated with radiotherapy (e.g., gastric ulcer) occurred.
In the analysis of toxicity associated with three fractionation schemes, grade 1-2 leukopenia significantly increased in the group that received a lowest fractional dose of 1.5 Gy (p=0.007). The occurrence rate of leukopenia was significantly higher in the 1.5-Gy group than in the 1.67-Gy and 1.8-Gy groups. This was likely to have occurred, given that the median treatment period was 23 days (range 21-27 days) in the 1.5-Gy group, longer than 19 days (range 17-23 days) in the 1.67-Gy group, and 19 days (range 13-22 days) in the 1.8-Gy group, and that leukopenia was more likely to have been detected from blood tests obtained from a longer treatment period. However, all of the patients with grade 1-2 leukopenia in the current study recovered spontaneously without bone marrow rescue injection. In the analysis for the correlation of fractionation schedule and GI toxicity, both Spearman's rho and partial correlation showed that toxicity significantly increased in the 1.8-Gy group. This result is consistent with the result of a previous study reporting that acute toxicity such as nausea increased as the fraction size increased in radiotherapy. In the current study, one third of the patients received a total dose of 25 Gy in 1.67 Gy per fraction over 3-week period. This fractionation schedule can shorten the overall treatment time compared to a 1.5-Gy-per-fraction schedule and reduce acute toxicity compared to a 1.8-Gy-per-fraction schedule. However, further study is required to determine optimal dose, treatment duration, and total dose for early testicular seminoma based on the results of the current study.
Although there have been controversies over the use of dog-leg field and PA field, more centers have accepted the PA field.8,17 The PA field has been used at our institution since 2003. Although the PA field has a relatively short follow-up period compared to the DL field, no pelvic recurrence occurred in the current study. In the analysis of acute toxicity, an increased rate of GI toxicity such as nausea was observed in the DL field group compared to the PA field group. Thus, the PA field can be considered as a safe and effective field for early testicular seminoma.
One limitation of this study was that most of the patients did not undergo semen analysis before and after radiotherapy. Thus, information regarding late radiation-induced complications such as reduced sperm production and infertility was not available. In a Southwest Oncology Group study (SWOG-8711) that was conducted to investigate reproductive function in 207 patients with testicular seminoma after radiotherapy, sperm concentration returned to normal levels within one year after radiotherapy in the patients who underwent radiotherapy at a testicular dose of <0.8 Gy and used clamshell-type shields for maximum protection.18 In this study, most of the patients were young males, and a clamshell-type shield was used in the opposite testis for the prevention of infertility during the treatment.
The result of this single-institution study showed that patients with stage I seminoma could be safely treated with PA-only radiotherapy with no pelvic failure after a median follow-up of 10 years. Optimal fraction schedule to minimize acute and chronic toxicity needs to be explored further.
Figures and Tables
Table 1
Patient & Treatment Characteristics

Table 2
Acute Toxicities according to Radiotherapy Volume and Fraction Size

References
1. McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003; 97:63–70.

2. Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol. 2003; 170:5–11.

3. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96.

4. Tandstad T, Smaaland R, Solberg A, Bremnes RM, Langberg CW, Laurell A, et al. Management of seminomatous testicular cancer: a binational prospective population-based study from the Swedish norwegian testicular cancer study group. J Clin Oncol. 2011; 29:719–725.

5. Groll RJ, Warde P, Jewett MA. A comprehensive systematic review of testicular germ cell tumor surveillance. Crit Rev Oncol Hematol. 2007; 64:182–197.

6. Oliver RT, Mason MD, Mead GM, von der Maase H, Rustin GJ, Joffe JK, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005; 366:293–300.

7. Pectasides D, Pectasides E, Constantinidou A, Aravantinos G. Stage I testicular seminoma: management and controversies. Crit Rev Oncol Hematol. 2009; 71:22–28.

8. Niazi TM, Souhami L, Sultanem K, Duclos M, Shenouda G, Freeman C. Long-term results of para-aortic irradiation for patients with stage I seminoma of the testis. Int J Radiat Oncol Biol Phys. 2005; 61:741–744.

9. Jones WG, Fossa SD, Mead GM, Roberts JT, Sokal M, Horwich A, et al. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I Testicular Seminoma: a report on Medical Research Council Trial TE18, European Organisation for the Research and Treatment of Cancer Trial 30942 (ISRCTN18525328). J Clin Oncol. 2005; 23:1200–1208.

10. Kamba T, Kamoto T, Okubo K, Teramukai S, Kakehi Y, Matsuda T, et al. Outcome of different post-orchiectomy management for stage I seminoma: Japanese multi-institutional study including 425 patients. Int J Urol. 2010; 17:980–987.

11. Mead GM, Fossa SD, Oliver RT, Joffe JK, Huddart RA, Roberts JT, et al. Randomized trials in 2466 patients with stage I seminoma: patterns of relapse and follow-up. J Natl Cancer Inst. 2011; 103:241–249.

12. Travis LB, Curtis RE, Storm H, Hall P, Holowaty E, Van Leeuwen FE, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997; 89:1429–1439.

13. Girinsky T, Ghalibafian M. Radiotherapy of hodgkin lymphoma: indications, new fields, and techniques. Semin Radiat Oncol. 2007; 17:206–222.

14. Koshy M, Rich SE, Mahmood U, Kwok Y. Declining use of radiotherapy in stage I and II Hodgkin's disease and its effect on survival and secondary malignancies. Int J Radiat Oncol Biol Phys. 2012; 82:619–625.

15. Kleinschmidt K, Dieckmann KP, Georgiew A, Loy V, Weissbach L. Chemotherapy is of limited efficacy in the control of contralateral testicular intraepithelial neoplasia in patients with testicular germ cell cancer. Oncology. 2009; 77:33–39.

16. Powles T, Robinson D, Shamash J, Moller H, Tranter N, Oliver T. The long-term risks of adjuvant carboplatin treatment for stage I seminoma of the testis. Ann Oncol. 2008; 19:443–447.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download