Abstract
Purpose
The aim of the present study was to investigate associations between the renin gene (REN) and the risk of essential hypertension and blood pressure (BP) levels in Koreans.
Materials and Methods
To outline the functional role of a single nucleotide polymorphism in the transcription of the REN gene, we conducted a case-control study of 1975 individuals: 646 hypertension (HT) patients and 1329 ethnically and age-matched normotensive subjects.
Results
Logistic regression analysis indicated that the genotypes AA/AG were strongly associated with risk of HT (odds ratio, 1.493; 95% confidence interval, 1.069-2.086, p=0.018) in female subjects. The genotypes AA/AG also showed significant association with higher blood pressure levels, both systolic and diastolic, in postmenopausal HT women (p=0.003 and p=0.017, respectively). Analysis of the promoter containing rs6682082 revealed a 2.4±0.01-fold higher activity in the A variant promoter than the G variant promoter, suggesting that rs6682082 is itself a functional variant.
Hypertension is a major risk factor for cardiovascular diseases, such as stroke, myocardial infarction, heart failure, and vascular disease. The early screening and identification of patients at risk is essential for preventing target organ damage and reducing mortality. Hypertension is considered a multi-factorial disease since its development is affected by both genetic and environmental factors.1,2
Intense efforts have been focused on identifying gene(s) related to hypertension. Recently, large-scale genome-wide association studies have uncovered an association between hypertension and/or blood pressure and several genes not previously identified.3,4,5 Among the single-nucleotide polymorphisms (SNPs) shown to be associated with blood pressure or hypertension, ATP2B1 and CYP17A1, as well as a few others, were found to be associated with the phenotype in different ethnic groups, while some of them were not.6 Surprisingly, genome-wide association studies have not found associations between renin-angiotensin system (RAS) genes and hypertension or blood pressure, despite extensive study.7
Candidate-gene-based association studies are still popular for identifying genetic components of essential hypertension (EH), and the genes of the RAS have been extensively studied as candidate genes associated with EH. Renin, a protease expressed mainly in the juxtaglomerular cells of the kidney, cleaves angiotensinogen to angiotensin I, which is in turn further processed to angiotensin II by angiotensin converting enzyme I. Angiotensin II is a potent vasoconstrictor and stimulates the release of aldosterone and vasopressin, thus contributing to an increase in blood pressure. Renin catalyzes the first and rate-limiting step of the RAS cascade, thereby playing a crucial role in the regulation of blood pressure and electrolyte homeostasis.8 Human renin gene (REN) is composed of 12 exons dispersed across 12 kb of human chromosome 1q32. Several polymorphic markers within the renin gene have been investigated for their associations with EH and blood pressure in various ethnic groups, showing contradictory results. Whereas many studies have shown negative associations between renin polymorphisms and EH,5,9,10,11 positive associations have also been reported in several ethnic groups, including two independent United Emirates populations, a Caucasian group in the USA, and two large Spanish populations, as well as Han, Tibetan, Mongolian, Indian, and a few other population groups.12,13,14,15,16,17,18
The expression of the renin gene is tightly regulated at the transcriptional level and its regulation is genetically controlled. Transcription of the human REN gene is dependent on three regions: a proximal promoter region, a tissue-specific element region, and an enhancer region.19 In the proximal promoter region of the human REN gene, various cis-acting elements have been identified, including a cAMP response element, a Pit-1 consensus binding site, an Ets-motif-like site, a HOX-PBX recognition sequence, COUP-TFII-motif-like sites, and a hormone responsive element.20,21,22 Mutations in some of these elements modulate the expression of renin.23
Several SNPs have been identified in the promoter and enhancer regions of the human REN gene.24,25 Among those, only one (rs12750834) has been shown to affect the transcription of REN.19 Recently, a few SNPs in the 5' upstream region of the REN gene have been shown to be positively associated with EH. However, the relationship between the renin expression and these polymorphisms is unclear.19
In the present study, we sought to determine the genetic contribution of renin to essential hypertension in Koreans and identified a positive association between the rs6682082 polymorphism in the promoter of the REN gene and EH, as well as increased blood pressure levels, in Korean women. We also demonstrated the functional role of this SNP in the transcription of the REN gene using a heterologous expression system. Based on these data, we discussed the relationship between the genetic variant and its molecular biological function in regards to EH in Korean women.
The 1975 individuals, 646 EH patients and 1329 normotensive subjects, who participated in this study were recruited by the Cardiovascular Genome Center of Yonsei University and the National Genome Research Institute of Korea. Hypertension patients were defined as those with a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg or those who were being administered antihypertensive agents. Patients with secondary hypertension, renal failure, diabetes mellitus, or significant hepatic disease or who were on corticosteroid therapy were excluded. Normotensives were defined as those with a blood pressure <140/90 mm Hg without a history of hypertension, renal insufficiency, significant hepatic disease, diabetes, or apparent coronary artery disease. The Ethics Committees of Yonsei University, as well as The Catholic University of Korea, approved this project, and all participants gave written, informed consent. All demographic data were collected via medical records and direct measurement by blood chemistry.
Genomic DNA was extracted from peripheral blood using a commercial genomic DNA purification kit (Promega, Madison, WI, USA). Genotypes rs6682082, rs11240688, rs10900555, rs2272237, rs1464816, and rs2368564 were determined by a single-base primer extension assay using the ABI PRISM SNaPshot kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's recommendation. Primer sequences for rs6682082 were as follows: forward 5'-TGT TTC CCA GCC TAA AAT AAT-3', reverse 5'-ACA GGT TAT CTA AAT GGG CTT C-3'; probe: 5'-TCA CAC TAC AGA AAG TTT TTC TTT G-3'. The genotyping of rs6682082 was carried out using an ABI prism 3730XL DNA analyzer.
The statistical analyses were carried out using the SAS program (version 9.2, SAS Institute Inc., Cary, NC, USA). Linkage dysequilibrium (LD) was calculated using Haploview 4.2. Analyses of clinical characteristics were carried out using a normality test and Student's t-test. Student's t-test and χ2 analyses were used to compare the mean values between groups for continuous or categorical measurements, respectively. The Hardy-Weinberg equilibrium (HWE) was assessed by χ2 analysis. Frequencies of genotype and alleles were compared using χ2 tests or Fisher's exact test. The relationship between genotypes and the risk of EH was reported in odds ratios (ORs), which were calculated with 95% confidence intervals (CIs). The OR was adjusted for body mass index (BMI), creatinine, triglyceride (TG), high-density lipoprotein (HDL), and glucose (Glu). A value of p<0.05 was considered statistically significant. The relationship between the genotypes of rs6682082 and blood pressure was analyzed using Student's t-test and Wilcoxon signed rank test. Blood pressure was presented as the mean±standard error.
A 2869 bp product of the proximal region of the human renin gene promoter was amplified from genomic DNA by PCR using the primers Renin F (5'-CTT GGT AGG ATC CCT GTG GCT A-3') and Renin R (5'-CTC AGT CTG GGG CTC TCT CTG-3'). The PCR product was cloned into pGEM-Teasy (Promega, Madison, WI, USA), and an EcoRI restriction fragment of this construct was inserted into the EcoRI-digested pGLuc basic vector (New England Biolabs, Beverly, MA, USA). This yielded a construct with the firefly luciferase reporter gene under control of the human renin gene promoter. Genomic DNAs from female homozygotes for the A allele or G allele were used for amplification of the Ren promoter region. To generate a G (A) promoter plasmid that contained the G type sequence across the whole promoter region, with the exception of A at the rs6682082 polymorphic site, we performed site-directed mutagenesis using the QuikChange site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA), as recommended by the manufacturer, with the following primers, 5'-TTA GGC CAG CTA CCA AAA ACG CAA AGA AAA ACT TTC TGT AG-3' and 5'-CTA CAG AAA GTT TTT CTT TGC GTT TTT GGT AGC TGG CCT AA-3'. PCR reaction was carried out in a 20 µL reaction mixture containing 10 ng of the original G type plasmid. The positive clone was verified by DNA sequence analysis.
The promoter activity was determined by the luciferase activity of the cells transfected with each plasmid. As 4.1 cells were grown in DMEM supplemented with 10% FBS, 100 µg/mL penicillin, and 100 µg/mL streptomycin at 37℃ in a humidified 5% CO2 incubator. Five hundred nanograms of the plasmid DNA was transiently co-transfected with 100 ng of beta-galactosidase-expression vector into 1×105 cells in a six-well culture plate using polyethylenimine.26 Twenty-four hours later, the medium was used to measure luciferase activity using BioLux™ Gaussian Luciferase Assay kit (NEB, Beverly, MA, USA), following the manufacturer's manual, and a Luminometer (Turner Designs, Promega, Madison, WI, USA). Luciferase activity was normalized against transfection efficiency determined by β-galactosidase activity. The promoter activity was obtained by subtracting the background activity of the pGLuc basic vector. Two independent experiments were performed in duplicate. The promoter activity was presented as the mean±standard error. The fold difference was calculated against the G type promoter activity. Data were compared by two-tailed Student's t-test.
A total of 1975 individuals, 646 EH patients and 1329 normotensive subjects, participated in this study, and their characteristics are presented in Table 1. The hypertensive group exhibited significantly higher systolic and diastolic blood pressures. There were significant differences between the HT and NT groups in their BMI and levels of creatinine, triglycerides (TG), HDL, and glucose (Glu), whereas age and total cholesterol and low-density lipoprotein levels did not differ between the two groups in either the male or female participants.
We prescreened 184 subjects (92 cases and 92 controls) for association with EH in our study population. They were genotyped for rs6682082, rs11240688, rs10900555, rs2272237, rs1464816, and rs2368564, which were selected based on their relative position on the renin gene, as well as their frequencies as described in the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/GeneGt.cgi?rpttype=Ldplot&geneID=5972&chr=1&popID=1411) for Asian populations (Supplementary Table 1, only online). Six SNPs were not in LD in Koreans (Supplementary Fig. 1, only online). Among the six SNPs investigated, we further investigated rs6682082 only, using the expanded study population, because only rs6682082 displayed a marginal tendency for the association with EH (p=0.149). We genotyped all 1975 subjects for the rs6682082 SNP. The observed and expected genotypes agreed well with the predicted HWE in all groups, with the exception of the female hypertensive group (p=0.025).
To determine whether an association existed between rs6682082 and EH, we used Fisher's exact test and χ2 analysis; the results are shown in Table 2. The overall genotype distribution and allele frequencies of rs6682082 differed significantly between the HT and NT groups. Specifically, the frequency of the A allele was much higher in the HT group than the NT group (0.135 vs. 0.111, respectively; p=0.028) when the whole study group was analyzed. This significant difference remained in the female group only when the study population was subdivided based on sex (Table 2). The frequency of the A allele was higher in the HT group than in the NT group (0.126 vs. 0.096, respectively; p=0.038), and the frequency of the AG/AA genotype was also higher in the hypertensive group (0.250 vs. 0.185, respectively; p=0.015).
A logistic regression analysis revealed that the OR for hypertension in subjects with the AG or AA genotype was 1.454 (95% CI=1.047-2.018) in the female participants. This OR became 1.493 (95% CI=1.069-2.086) after adjustments were made for confounding factors, including BMI, TG, HDL, creatinine, and Glu (Table 3). Therefore, the genotype of the rs6682082 polymorphism was associated with the risk of EH in the female participants (p=0.018), and the A allele was an independent positive risk factor for EH in female Koreans.
We also analyzed the relationship between blood pressure and the rs6682082 genotype. We included both NT and EH individuals in this analysis and used blood pressure levels measured before any antihypertensive drug treatment for the EH patients. Since the genetics of female patients with EH significantly differ before and after menopause, further analysis was carried out with the participants whose menopause statuses were known. These included 332 and 448 participants in pre- and post-menopause, respectively. A significant relationship between the rs6682082 genotype and blood pressure was found in post-menopausal women and this relationship was prominent in EH patients. In postmenopausal EH patients, the mean systolic blood pressure (SBP) was 168.05±2.95 mm Hg for participants with the AA/AG genotype and 160.57±1.50 mm Hg for those with the GG genotype (p=0.003). Likewise, the mean diastolic blood pressure (DBP) was 105.32±2.37 mm Hg for participants with the AA/AG genotype and 100.39±1.14 mm Hg for those with the GG genotype (p=0.017) in postmenopausal EH patients. Thus, the AA/AG genotype was associated with significantly higher SBP and DBP than the GG genotype in postmenopausal EH women (Fig. 1).
To understand the mechanism underlying the association between rs6682082 and EH, we investigated the functional role of this SNP at the molecular level. As rs6682082 is located in the promoter region of the REN gene, we investigated the effect of this SNP on REN transcription. We compared the activities of the promoters containing the A or G nucleotide in rs6682082 using a luciferase reporter assay, as described in the Methods section. The luciferase activity of the A-type promoter was 2.4±0.01 fold higher than that of the G-type promoter (Fig. 2). Furthermore, the activity of the G (A) promoter, which is the G-type promoter with an A at the rs6682082 position, was similarly higher (2.0±0.4 fold) than that of the G-type promoter. These results suggest that the transcription of the REN gene is differentially regulated depending on the nucleotide variant of rs6682082 and that this SNP itself directly affects the transcriptional activity
of the REN gene (Fig. 2).
Renin plays a pivotal role in the regulation of blood pressure27 and elevated renin levels are a risk factor for EH. Although renin acts as the rate-limiting step of the RAS cascade, renin has attracted less research attention than other candidate proteins in terms of its genetic association with EH. Recent development of a renin inhibitor as an antihypertensive drug has rekindled interest in the association between renin polymorphisms and EH.
There were several genetic markers of REN that have shown positive associations with EH and a few in the promoter region in different ethnic groups.14,18,28 The rs6696954 and rs5705 showed association with EH in Caucasians and the intronic BglI site with EH in a United Arab Emirates population.13,18 The rs41317140 (Taq1, C-4036T) in the 5' flanking region of REN showed discrepancies in association with EH in different ethnic groups29: it was positively associated with EH in a Han population, while no association was found in Bangladeshi patients with EH.11
Some renin markers showed significant association with elevated blood pressure. rs12750834 (C-5312T) was associated with elevated blood pressure in Caucasians.24
The present study found an association of rs6682082 with hypertension and blood pressure in post-menopausal women. Interestingly, rs5707 was shown to be associated with high blood pressure in post-menopausal Spanish women.12 The rs6682082 and rs5707 polymorphisms are located in the promoter and intron 6 of REN, respectively. In order to determine whether these two markers are linked to each other, we analyzed LD status between the two SNPs using Haploview 4.2 in HapMap Asian populations (HapMap-JPT and HapMap-HCB), as there was no genotype data available for rs5707 in Koreans. SNPs rs6682082 and rs5707 were not noted in LD in both Japanese and Chinese (r2=zero) populations, suggesting that they may not be found in LD in Koreans either. Thus, rs6682082 is an independent genetic marker for EH and BP in Korean women, and associations of rs5707 with hypertension and blood pressure can be assessed in Korean populations (Supplementary Fig. 2, only online).
Among recent studies of RAS genes for association with hypertension,3,4,16,30 rs6682082 has been under consideration in a single study, in which no association was found therewith and uncontrolled arterial hypertension in an ethnic Spanish group.30 In 2011, Song, et al.3 identified RAS-related genes that were associated with BP in the Korea Association Resource subject pool. They analyzed correlations between BP and 12 REN SNPs (rs6679960, rs4951307, rs3737656, rs1997034, rs11571093, rs2887284, rs2368564, rs11571082, rs11571103, rs10900555, rs6693954, and rs16853059), which showed no association. The reason why none of these SNPs showed any association with BP in Korean women is not clear. One reason may be that the authors might not have analyzed for gender-specific associations. Another possibility is that none of these markers is linked to rs6682082. The closest marker to rs6682082 is rs6693954, which is located 4.3 kb away from rs6682082. Although we do not know the LD status of these markers in Koreans, they are not found in LD in Japanese and Chinese populations, suggesting that these are not in LD in Koreans (Haploview Supplementary Fig. 2, only online).
In the present study, we identified a new independent genetic marker, rs6682082, as being strongly associated with risk of EH and high BP in Korean women. Blood pressure tends to increase with age, more so for women than men. Especially after menopause, risk of EH for women increases greatly, thus leading to more female than male EH patients
among individuals older than 55 years. Thus, with increasing life expectancy after menopause, identifying risk groups for potential hypertension among women is important to addressing the threat of cardiovascular disorders, and rs6682082 can provide a means for pre-symptomatic diagnosis. Thus, in the light of the aging Korean society, with more people older than 55 years than younger, rs6682082 may be useful in diagnosing EH in Korean females.
Postmenopausal-related increase in BP has been attributed to a variety of factors, including the renin angiotensin system, estrogen/androgen ratio, endothelial dysfunctions, oxidative stress, obesity, and type II diabetes.31,32 In our study, rs6682082, which affects renin expression, was found to be associated with BP in Korean postmenopausal women, suggesting that the RAS system must be involved in BP regulation in postmenopausal women. However, losartan, an angiotensin type 1 receptor blocker, failed to restore normal BP in postmenopausal rats, although it significantly reduced BP in those rats.33 Thus, further study is required to better understand factors contributing to postmenopause-related BP increases and EH in Korean women.
The underlying mechanisms by which polymorphisms contribute to the development of EH have not been explored. We found that the A allele of rs6682082 showed much greater promoter activity than the G allele when it was assessed by using a luciferase reporter system. Interestingly, the A allele promoter that displayed the greatest promoter activity was found to be a risk factor for EH in Korean women, suggesting that genetic differences may affect the expression of renin and thereby contribute to hypertension.
Promoter activity is modulated by the interaction of transcription factors with their responsive elements in the DNA sequence of the promoter region. We searched for transcription factors (TF) that might bind to the sequence containing rs6682082 using publicly available transcription binding factor prediction programs (TOMTOM; http://meme.nbcr.net/meme/cgi-bin/tomtom.cgi and CONSITE; http://asp.ii.uib.no:8090/cgi-bin/CONSITE/consite): Runt-related transcription factor 1 (RUNX1), SRY-related HMG-box family (Sox family), and a few other factors were identified. RUNX1 plays a role in hematopoiesis, and Sox family TFs are involved in sex determination and cell fate determination in numerous developmental processes.34 Currently, neither factors have been shown to be associated with HT or BP regulation, and no known TF was found to be associated with HT or BP regulation in our search. Therefore, factors binding to rs6682082 present an opportunity to identify a new TF that regulates REN expression and affects BP level, especially in females.
Our study has a few limitations. First, the statistical power of this study was limited due to the restricted sample size. Second, promoter activity was not supported with endogenous plasma renin levels. Although these limits somewhat weaken the power of this study, it is clear that rs66820982 is an important independent marker for EH in Korean women.
In conclusion, we identified the A allele of the rs6682082 SNP in human REN as a risk factor for EH in Korean women and suggested a possible mechanism for how this risk factor may contribute to the development of EH. Via our study, we identified a new regulatory region in REN promoter that influences transcription of the REN gene. Although an SNP (rs5707) has previously been reported to be associated with EH and high BP in post-menopausal women, rs6682082 is the first SNP to show both functional relevance and associations with EH and high BP. Furthermore, this is the first report to show a positive genetic risk factor for EH in the renin gene of Koreans.
Figures and Tables
Fig. 1
Differences in systolic blood pressure (SBP) and diastolic blood pressure (DBP) by rs6682082 genotype in postmenopausal EH patients. (A) Means of SBP were 168.05±2.95 mm Hg in participants with the AA/AG genotype and 160.57±1.50 mm Hg in participants with the GG genotype (p=0.003). (B) Means of DBP were 105.32±2.37 mm Hg in participants with the AA/AG genotype and 100.39±1.14 mm Hg in participants with the GG genotype (p=0.017). BP was described by mean±standard error. EH, essential hypertension.

Fig. 2
Luciferase activity of AS 4.1 cells transfected with renin SNP rs6682082/luciferase gene constructs. The G (A) promoter construct was prepared by site-directed mutagenesis of the G allele promoter construct. The relative luciferase activity is presented by mean±standard error. *A allele vs. G allele, †G (A) allele vs. G allele. SNP, single-nucleotide polymorphism.

Table 1
Clinical Characteristics of the Participants in the Study Population
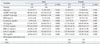
NT, normotensives; HT, hypertensives; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; TG, triglyceride; TCHOL, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Glu, glucose.
Data values are means±standard deviation and numbers. Age difference was not significant. p-value was estimated by Student's t-test.
*SBP and DBP were BP prior to medication.
Table 2
Renin rs6682082 Genotype/Allele Distribution in the Study Population

ACKNOWLEDGEMENTS
This study was supported by a grant of Korean Health 2001 R&D project, Ministry of Health and Welfare, Republic of Korea (HMP-00-GN-01-0001).
References
1. Williams FM, Cherkas LF, Spector TD, MacGregor AJ. A common genetic factor underlies hypertension and other cardiovascular disorders. BMC Cardiovasc Disord. 2004; 4:20.

2. Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet. 2003; 361:1629–1641.

3. Song SB, Jin HS, Hong KW, Lim JE, Moon JY, Jeong KH, et al. Association between renin-angiotensin-aldosterone system-related genes and blood pressure in a Korean population. Blood Press. 2011; 20:204–210.

4. Morales-Suárez-Varela MM, Mansego ML, Vicedo-Cabrera AM, Pineda-Alonso M, Llopis-González A, Martin-Moreno JM, et al. Inefficient arterial hypertension control in patients with metabolic syndrome and its link to renin-angiotensin-aldosterone system polymorphisms. Hypertens Res. 2011; 34:758–766.

5. Ji L, Cai X, Zhang L, Fei L, Wang L, Su J, et al. Association between polymorphisms in the renin-angiotensin-aldosterone system genes and essential hypertension in the Han Chinese population. PLoS One. 2013; 8:e72701.

6. Kelly TN, Takeuchi F, Tabara Y, Edwards TL, Kim YJ, Chen P, et al. Genome-wide association study meta-analysis reveals transethnic replication of mean arterial and pulse pressure loci. Hypertension. 2013; 62:853–859.

7. Ichikawa M, Konoshita T, Nakaya T, Yamamoto K, Yamada M, Sato S, et al. Genetic variant of the renin-angiotensin system and prevalence of type 2 diabetes mellitus: a modest but significant effect of aldosterone synthase. Acta Diabetol. 2014; 51:595–599.

8. Griendling KK, Murphy TJ, Alexander RW. Molecular biology of the renin-angiotensin system. Circulation. 1993; 87:1816–1828.

9. Morris BJ, Griffiths LR. Frequency in hypertensives of alleles for a RFLP associated with the renin gene. Biochem Biophys Res Commun. 1988; 150:219–224.

10. Jeunemaitre X, Rigat B, Charru A, Houot AM, Soubrier F, Corvol P. Sib pair linkage analysis of renin gene haplotypes in human essential hypertension. Hum Genet. 1992; 88:301–306.

11. Afruza R, Islam LN, Banerjee S, Hassan MM, Suzuki F, Nabi AN. Renin gene polymorphisms in bangladeshi hypertensive population. J Genomics. 2014; 2:45–53.

12. Mansego ML, Redon J, Marin R, González-Albert V, Martin-Escudero JC, Fabia MJ, et al. Renin polymorphisms and haplotypes are associated with blood pressure levels and hypertension risk in postmenopausal women. J Hypertens. 2008; 26:230–237.

13. Frossard PM, Lestringant GG, Malloy MJ, Kane JP. Human renin gene BglI dimorphism associated with hypertension in two independent populations. Clin Genet. 1999; 56:428–433.

14. Qi Y, Niu W, Cen W, Cui C, Zhuoma C, Zhuang L, et al. Strong association of the renin TaqI polymorphism with essential hypertension in Chinese Han and Tibetan populations. J Hum Hypertens. 2007; 21:907–910.

15. Ying CQ, Wang YH, Wu ZL, Fang MW, Wang J, Li YS, et al. Association of the renin gene polymorphism, three angiotensinogen gene polymorphisms and the haplotypes with essential hypertension in the Mongolian population. Clin Exp Hypertens. 2010; 32:293–300.

16. Mohana Vamsi U, Swapna N, Usha G, Vishnupriya S, Padma T. Contribution of REN gene MBbo I polymorphism in conferring risk for essential hypertension: a case control study from South India. J Renin Angiotensin Aldosterone Syst. 2013; 14:242–247.

17. Niu W, Qi Y, Guo S, Gao P, Zhu D. Association of renin BglI polymphism with essential hypertension: a meta-analysis involving 1811 cases and 1626 controls. Clin Exp Hypertens. 2010; 32:431–438.

18. Sun B, Williams JS, Pojoga L, Chamarthi B, Lasky-Su J, Raby BA, et al. Renin gene polymorphism: its relationship to hypertension, renin levels and vascular responses. J Renin Angiotensin Aldosterone Syst. 2011; 12:564–571.

19. Fuchs S, Philippe J, Germain S, Mathieu F, Jeunemaitre X, Corvol P, et al. Functionality of two new polymorphisms in the human renin gene enhancer region. J Hypertens. 2002; 20:2391–2398.

20. Borensztein P, Germain S, Fuchs S, Philippe J, Corvol P, Pinet F. cis-regulatory elements and trans-acting factors directing basal and cAMP-stimulated human renin gene expression in chorionic cells. Circ Res. 1994; 74:764–773.

21. Konoshita T, Makino Y, Wakahara S, Ido K, Yoshida M, Kawai Y, et al. Candidate cis-elements for human renin gene expression in the promoter region. J Cell Biochem. 2004; 93:327–336.

22. Germain S, Konoshita T, Philippe J, Corvol P, Pinet F. Transcriptional induction of the human renin gene by cyclic AMP requires cyclic AMP response element-binding protein (CREB) and a factor binding a pituitary-specific trans-acting factor (Pit-1) motif. Biochem J. 1996; 316(Pt 1):107–113.

23. Germain S, Konoshita T, Fuchs S, Philippe J, Corvol P, Pinet F. Regulation of human renin gene transcription by cAMP. Clin Exp Hypertens. 1997; 19:543–550.

24. Moore N, Dicker P, O'Brien JK, Stojanovic M, Conroy RM, Treumann A, et al. Renin gene polymorphisms and haplotypes, blood pressure, and responses to renin-angiotensin system inhibition. Hypertension. 2007; 50:340–347.

25. Itani HA, Liu X, Pratt JH, Sigmund CD. Functional characterization of polymorphisms in the kidney enhancer of the human renin gene. Endocrinology. 2007; 148:1424–1430.

26. Kim BK, Lee HY, Choi JH, Kim JK, Yoon JB, Yoon SK. Hairless plays a role in formation of inner root sheath via regulation of Dlx3 gene. J Biol Chem. 2012; 287:16681–16688.

27. Tanimoto K, Sugiura A, Kanafusa S, Saito T, Masui N, Yanai K, et al. A single nucleotide mutation in the mouse renin promoter disrupts blood pressure regulation. J Clin Invest. 2008; 118:1006–1016.

28. Niu W, Qi Y, Hou S, Zhai X, Zhou W, Qiu C. Haplotype-based association of the renin-angiotensin-aldosterone system genes polymorphisms with essential hypertension among Han Chinese: the Fangshan study. J Hypertens. 2009; 27:1384–1391.

29. Barley J, Carter ND, Cruickshank JK, Jeffery S, Smith A, Charlett A, et al. Renin and atrial natriuretic peptide restriction fragment length polymorphisms: association with ethnicity and blood pressure. J Hypertens. 1991; 9:993–996.

30. Morales-Suárez-Varela MM, Mansego ML, Martín-Escudero JC, Llopis-González A, Chaves FJ, López-Izquierdo R, et al. How ineffective hypertension control in subjects treated with angiotensin-converting enzyme inhibitors is related to polymorphisms in the renin-angiotensin-aldosterone system. Eur J Pharm Sci. 2010; 39:380–386.

31. Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008; 51:952–959.
32. Reckelhoff JF, Fortepiani LA. Novel mechanisms responsible for postmenopausal hypertension. Hypertension. 2004; 43:918–923.

33. Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. Postmenopausal hypertension: role of the Renin-Angiotensin system. Hypertension. 2010; 56:359–363.
Supplementary Material
Supplementary Table 1
Genotype and Allele Frequencies of the SNPs in Asian Populations (HapMap-JPT and HapMap-HCB) and Our Study Population
Supplementary Fig. 1
Diagram of the renin gene and linkage disequilibrium structure of single nucleotide polymorphisms (rs6682082, rs11240688, rs10900555, rs2272237, rs1464816, and rs2368564).
Supplementary Fig. 2
Linkage disequilibrium of renin SNP in East Asian populations, (A) HapMap-JPT and (B) HapMap-HCB (1:rs11571093, 2:rs11571123, 3:rs11571091, 4:rs2887284, 5:rs2368564, 6:rs5706, 7:rs3795575, 8:rs11571120, 9:rs11571119, 10:rs3730101, 11:rs1157, 12:rs11571117, 13:rs11571116, 14:rs11571114, 15:rs5707, 16:rs11571083, 17:rs11571083, 18:rs11571082, 19:rs5705, 20:rs11571108, 21:rs11571080, 22:rs11571107, 23:rs11571104, 24:rs11471103, 25:rs10900555, 26:rs6693954, 27:rs11571102, 28:rs11571079, 29:rs6676670, 30:rs11571078, 31:rs11571101, 32:rs11571100, 33:rs11240688, 34:rs11571098, 35:rs6668858, 36:rs11571097, 37:rs6681776, 38:rs6682082). SNP, single-nucleotide polymorphism.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download