This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
The objective of this study was to evaluate our institutional experience with veno-venous (VV) extracorporeal membrane oxygenation (ECMO) in patients with severe acute respiratory failure (ARF).
Materials and Methods
From January 2007 to August 2013, 31 patients with severe ARF that was due to various causes and refractory to mechanical ventilation with conventional therapy were supported with VV ECMO. A partial pressure of arterial oxygen (PaO2)/inspired fraction of oxygen (FiO2) <100 mm Hg at an FiO2 of 1.0 or a pH <7.25 due to CO2 retention were set as criteria for VV ECMO.
Results
Overall, 68% of patients survived among those who had received VV ECMO with a mean PaO2/FiO2 of 56.8 mm Hg. Furthermore, in trauma patients, early use of ECMO had the best outcome with a 94% survival rate.
Conclusion
VV ECMO is an excellent, life-saving treatment option in patients suffering from acute and life-threatening respiratory failure due to various causes, especially trauma, and early use of VV ECMO therapy improved outcomes in these patients.
Keywords: Acute respiratory failure, extracorporeal membrane oxygenation, mechanical ventilation, survival rate
INTRODUCTION
Acute respiratory failure (ARF) is a severe and life-threatening reaction to injuries or acute infections of the lung. The most severe form of the disease causes hypoxemia, characterized by partial pressure of arterial oxygen (PaO
2)/inspired fraction of oxygen (FiO
2) <100 mm Hg. The prognosis of this most severe form of ARF is dismal; the mortality rate exceeds 60%.
1,
2
During the management of ARF, mechanical ventilation with conventional therapies is usually the first step; however, severe forms of ARF may be refractory to this type of management. Moreover, high inspiratory pressure may deteriorate pulmonary function and irreversibly damage the lung.
3
Extracorporeal membrane oxygenation (ECMO) has the unique potential to support gas exchange and improve patient survival without causing further lung damage from invasive positive pressure ventilation in adult patients with fulminant respiratory failure.
4
The aim of this study was to present our institutional experience in evaluating the outcomes of veno-venous (VV) ECMO in patients with severe ARF due to various causes, in whom mechanical ventilation with conventional respiratory treatment could not provide adequate gas exchange.
MATERIALS AND METHODS
This study received approval from our Institutional Review Board (IRB No. 2012-92). Informed consent was not required due to the retrospective study design. The study reviewed the records of 31 patients who had received lung support by VV ECMO due to acute respiratory failure, from a total of 154 patients who had undergone ECMO due to acute cardiac failure or acute respiratory failure from August 2007 to August 2013 (
Fig. 1).
The mechanical ventilator was set to have a tidal volume of 5-6 mL/kg and a low peak inspiratory pressure (40 cmH2O or less). The criteria for VV ECMO included the treatment of mechanical ventilation and optimal conventional therapy with PaO2/FiO2 <100 at an FiO2 of 1.0, with >6 cm H2O of positive end-expiratory pressure (PEEP), or a pH <7.25 due to CO2 retention. The time interval before performing ECMO was the duration from the set time of an FiO2 of 1.0 to the time of the ECMO implant. Patients >75 years old and those with a terminal stage malignancy or uncontrollable bleeding were excluded. Three of the patients initially underwent veno-arterial ECMO due to cardiogenic shock or cardiac arrest, followed by VV ECMO after recovery of cardiac function yet without recovery of lung function. One patient who had both cardiogenic shock and ARF underwent veno-venoarterial (VVA) ECMO.
Three types of centrifugal pumps were used for ECMO. From 2007-May 2010, a Capiox Emergency Bypass System (Terumo, Inc., Tokyo, Japan) and a Bio-Pump (Medtronic Inc., Minneapolis, MN, USA) were used; after June 2010, a Centrifugal Rotaflow pump (Maquet Inc., Hirrlingen, Germany) was used. VA ECMO was performed using a 17-Fr arterial cannula (BioMedicus Medtronic Inc., Minneapolis, MN, USA) and a 21-Fr venous cannula (Biomedicus multistage femoral venous cannula, Medtronics Inc., Minneapolis, MN, USA), while a 21-Fr venous cannula (DLP: Medtronic Inc., Minneapolis, MN, USA) was used when switching to VV ECMO. VV ECMO was performed using 17-28-Fr venous cannulae (DLP: Medtronic Inc., Minneapolis, MN, USA or RMI: Edward's Lifescience LLC, Irvine, CA, USA) and a 21-Fr venous cannula (Biomedus multistage femoral venous cannula, Medtronic Inc., Minneapolis, MN, USA) for drainage. Two patients underwent catheter insertion in the intensive care unit, while catheter insertion in the remaining patients was completed in the cardiac catheterization laboratory using a 50-80 U/kg injection of heparin, followed by catheter insertion at the femoral artery and vein for VA ECMO and at both femoral veins for VV ECMO via the Seldinger method. For anticoagulation, patients without continuous renal replacement therapy and bleeding complications were managed with an activated clotting time set at 140-180 sec by 800-1000 U/h of heparin. In the remaining patients, partial thromboplastin time was set at 60-80 sec with 0.4-1.5 mg/kg/h of nafamostat mesilate (SK Chemicals Life Science Biz., Seoul, Korea licensed by Torii Pharmaceutical Co. Ltd., Tokyo, Japan).
ECMO flow was maintained at a mean blood pressure of >60 mm Hg with 3.0-4.0 L/min blood flow for VA ECMO patients with norepinephrine, as needed. For VV ECMO patients, SaO2 was maintained at >90% with a flow of 3.5-4.5 L/min. During ECMO, ventilators were set to a tidal volume of 5 mL/kg, a respiration rate of 10/min, a PEEP of 4-8 cmH2O, and an FiO2 of 0.21-0.6. Hematocrit >35% and platelets >50000-100000/mL were obtained with effort and transfusions performed when necessary. ECMO was removed when arterial blood gas analysis revealed pH 7.35-7.45, PaO2 >80 mm Hg, and PCO2 <45 mm Hg under the following conditions: a gas blender FiO2 of 0.21, sweep gas of 0 L/min at an ECMO flow of 2 L/min, and the ventilator mode set to an FiO2 of 0.6, a tidal volume of 6 mL/kg, a PEEP of 8 cmH2O, and an RR of 12-16/min for VV ECMO or 3 L/min of O2 via nasal prong with awakening ECMO patients. VA ECMO was exchanged for VV ECMO by removing the arterial cannula and inserting the venous cannula in the femoral vein on the opposite leg for patients who did not need VA ECMO support due to severe ARF.
Statistics were calculated using IBM SPSS 21.0 (IBM Corp., Armonk, NY, USA). Categorical variables are shown as percentages and analyzed by Pearson's χ2 test or Fisher's exact test. Continuous variables are shown as median (interquartile range) and analyzed by the Mann-Whitney U test. All p values were two sided, and p<0.05 was considered to indicate statistical significance.
RESULTS
The clinical features of the 31 patients who underwent ECMO due to ARF are shown in
Table 1. Of these patients, 25 were males with a median age of 48 years. Dividing patients by the type of ECMO, 27 patients (87%) received VV, 3 (10%) initially received VA followed by VV, and 1 (3%) received V-AV (
Table 1).
To determine risk factors for mortality, we compared the characteristics of the patients who survived to those that died (
Table 2). The patients who survived were younger, had traumatic ARF, and 13 (62%) had ECMO performed in the emergency room. In addition, the time interval before performing ECMO, the number of pre-ECMO mechanical ventilation days, and the ECMO duration were shorter, with a low-peak blood urea nitrogen (BUN) level (
p<0.05).
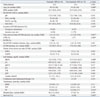
The traumatic ARF patient group had a higher survival rate than the non-traumatic patient group (
p<0.002), and we also analyzed the factors related to this outcome (
Table 3). The patients with traumatic ARF were young, and cardiopulmonary resuscitation and ECMO were performed primarily in the emergency room within a short period of time. In addition, the ECMO duration was short and the initial and peak BUN levels were low (
p<0.05).
The complications that occurred during ECMO included multi-organ failure in 6 patients, acute renal failure in 5 patients, bed sores in 3 patients, ulcer bleeding in 3 patients, and cholecystitis in 2 patients. We also recorded one incidence each of extra-cannula site bleeding, leg ischemia, bronchopleural fistula, cerebral infarction, and encephalitis. The causes of death were multi-organ failure for 6 patients (60%), encephalitis for 1 patient, and bronchopleural fistula for 1 patient; additionally the guardians of 2 patients refused further treatment (
Table 4).
Nineteen of the 21 patients who survived were followed up for a median of 5 months (range, 1-33 months) after discharge from hospital. Among these patients, one, who was diagnosed as brain dead, died 1 month after discharge, and another who was diagnosed with stage IV lung cancer died 14 months after discharge. Seventeen patients returned to their normal lives without complications.
DISCUSSION
Despite recent advances in critical care management, the mortality of ARF remains high. Patients were considered for VV ECMO in cases of potentially reversible acute and life-threatening respiratory failure. Recent studies suggest that VV ECMO may improve the outcomes of patients with severe ARF; however, indications for ECMO use remain uncertain,
5,
6,
7 leading to questions of which patients are the best candidates for ECMO and whether ECMO should be initiated early in the course of ARF or only in later stages of failure.
8 To answer these questions, the extracorporeal life support organization recommended guidelines for ECMO,
9 and numerous studies have reported their experiences and indications of ECMO for patients with severe ARF, in addition to evaluating the factors influencing patient survival.
10,
11,
12,
13
In this cohort, 31 patients with severe ARF did not respond to advanced respiratory treatment, with a mean PaO
2/FiO
2 of 56.8 mm Hg before ECMO. The overall survival rate in the patients was 68%. In comparing the survivor and non-survivor groups, patient age, pre-ECMO ventilation duration, time interval before performing ECMO, and ARF due to trauma were significantly different. Schmid, et al.
14 analyzed 176 patients with acute lung failure that was refractory to conventional therapies and was supported with VV ECMO. They reported an overall survival rate of 56%, and the risk factors affecting survival were advanced age and multiple organ failure, although duration of pre-ECMO mechanical ventilation was not a risk factor. Another study reported that advanced age, pre-ECMO pH <7.18, underlying cause of respiratory failure, and increased duration of pre-ECMO ventilation were associated with increased mortality, with an overall survival rate of 50%.
15 Similar to our results, several studies
15,
16,
17,
18,
19 have reported significant differences in the duration of pre-ECMO ventilation between survivors and non-survivors. Moreover, reports indicate the importance of considering ECMO early (<7 days) to prevent iatrogenic lung damage from high-pressure and high-FiO
2 ventilation.
20
Surprisingly, a 94% survival rate (15 of 16 patients) was achieved in our trauma patients among those who received a short pre-ECMO ventilation interval of 1.0 days. This result was superior to survival rates reported in previous studies. Cordell-Smith, et al.
21 reported that 20 of 28 patients (71%) who received ECMO with severe trauma-related respiratory failure survived, and the pre-ECMO ventilation times of survivors and non-survivors were 61 h and 87 h, respectively. Ried, et al.
22 evaluated 26 patients who received VV ECMO with severe trauma-related respiratory failure after 2.6 d of pre-ECMO ventilation and reported an 81% survival rate. Another study reported that the overall survival of 176 patients supported with VV ECMO was 56%, and the best outcome was noted in trauma patients (71%, 10 of 14 patients) whose pre-ECMO ventilation interval was 4.4 d.
14
Another important factor that could contribute to the high survival rate of our trauma patients was the shorter time interval before performing ECMO, which was 1.6 h after decreasing PaO2/FiO2 <100 mm Hg at an FiO2 of 1.0 or a pH level of <7.25 due to CO2 retention as indications for ECMO. In comparison, our non-trauma patients had a significantly different time interval before performing ECMO (6.1 h; p=0.001), with a survival rate of 40%. Furthermore, the time interval between survivors (2.2 h) and non-survivors (12.6 h) in our analysis was also significantly different (p=0.043). These results suggest that the time interval before performing ECMO could be one of the factors influencing survival, along with younger age and duration of pre-ECMO ventilation.
Several limitations must be considered in the interpretations of our results. First, this study was based on a process of a single-institution, which has a well-trained and experienced ECMO team. However, not all hospitals have an experienced ECMO team. Thus, this difference in resources is one of the limitations of our study, although we have highlighted early application of ECMO. Second, the study was not a randomized controlled trial, but rather retrospective analysis of our experience with VV ECMO due to various causes. Furthermore, the total sample size was small, and several causes had samples sizes of only 1 patient. In order to overcome these limitations, the patients were categorized into trauma and non-trauma groups for analysis, as well as survival and non-survival groups, to determine the factors that influence survival.
In conclusion, VV ECMO is an excellent and life-saving treatment option in patients suffering from acute and life-threatening respiratory failure due to various causes. Overall, 68% of patients survived. Furthermore, younger trauma patients, shorter pre-ECMO ventilation durations, and shorter time intervals before performing ECMO had the best outcomes with a 94% survival rate. Physicians should be aware of the factors that affect survival and consider early use of VV ECMO therapy to save lives.
Figures and Tables
 | Fig. 1Causes of acute respiratory failure. ECMO, extracorporeal membrane oxygenation; VA, venoarterial; ICH, Intracerebral hemorrhage.
|
Table 1
Baseline Clinical Characteristics of the Study Patients (n=31)

|
Age, yrs, median (IQR) |
48 (26, 55) |
|
Male (%) |
25 (80.6) |
|
BMI, median (IQR) |
23.2 (20.8, 24.9) |
|
Past medical history (%) |
|
|
Diabetes |
4 (13) |
|
Hypertension |
7 (23) |
|
Acute myocardial infarction |
1 (3) |
|
Liver cirrhosis |
2 (7) |
|
Chronic obstructive pulmonary disease |
2 (7) |
|
Asthma |
2 (7) |
|
Chronic renal disease |
1 (3) |
|
Locations of ECMO decision (%) |
|
|
Emergency department |
14 (45) |
|
Intensive care unit |
17 (55) |
|
CPR |
10 (32) |
|
ECPR |
1 (3) |
|
Surgical procedures (%) |
|
|
Surgical procedures with ECMO |
3 (10) |
|
ECMO after surgical procedures |
6 (19) |
|
Pre-ECMO ABGA, median (IQR) |
|
|
pH |
7.24 (7.08, 7.31) |
|
PO2, mm Hg |
58 (43, 64) |
|
PCO2, mm Hg |
57 (43, 75) |
|
PaO2/FiO2
|
58 (46.8, 64.3) |
|
Time interval before ECMO decision, hrs, median (IQR) |
3.1 (1.5, 6.7) |
|
Pre-ECMO mechanical ventilation duration, hrs, median (IQR) |
4 (2, 72) |
|
ECMO on admission day (%) |
22 (71) |
|
Within 24 hrs before and after ECMO, median (IQR) |
|
|
SOFA score |
12 (11, 15) |
|
SAPS 2 score |
55 (43, 69) |
|
Type of ECMO (%) |
|
|
Veno-venous |
27 (87) |
|
VA switched to VV |
3 (10) |
|
V-VA |
1 (3) |
|
Anticoagulation (%) |
|
|
Heparin |
8 (26) |
|
Nafamostat mesilate (%) |
23 (74) |
|
Awakening ECMO (%) |
4 (13) |
|
ECMO duration, hrs, median (IQR) |
166 (115, 306) |
|
Continuous renal replacement therapy (%) |
21 (68) |
|
Laboratory findings, mg/dL, median (IQR) |
|
|
iBUN |
16.2 (13.5, 22.4) |
|
iCr |
1.0 (0.8, 1.2) |
|
pBUN |
37.05 (23.55, 67.43) |
|
pCr |
1.05 (0.8, 1.723) |
|
iTB |
0.83 (0.47, 1.65) |
|
pTB |
3.0 (1.52, 5.71) |
|
Lactate, mmol/L |
5.5 (2.5, 9.0) |
|
Blood transfusion during ECMO, units/day, median (IQR) |
|
|
Packed red blood cells |
1.0 (0.7, 1.9) |
|
Fresh frozen plasma |
0.6 (0, 1.0) |
|
Platelet concentrate |
1.3 (0, 4.0) |
|
Hospital days, median (IQR) |
35 (21, 74) |
|
Outcomes (%) |
|
|
Death, including patients who refused further treatment |
5 (16) |
|
Weaned followed by death |
5 (16) |
|
Survival |
21 (68) |

Table 2
Clinical Characteristics of the Survival and Non-Survival Groups

|
Survivor (n=21) |
Non-survivor (n=10) |
p value |
|
Males:females |
16:5 |
9:1 |
0.634 |
|
Age, yrs, median (IQR) |
38 (20.5, 48) |
58 (51.8, 70.5) |
<0.001*
|
|
BMI, median (IQR) |
22.5 (21.0, 24.8) |
23.5 (19.3, 26.0) |
0.787 |
|
Pre-ECMO ABGA, median (IQR) |
|
|
|
|
pH |
7.24 (7.05, 7.28) |
7.26 (7.15, 7.38) |
0.268 |
|
PaO2, mm Hg |
58 (38, 64) |
53 (47, 64) |
0.852 |
|
PaCO2, mm Hg |
50 (41, 69) |
76 (55, 92) |
0.035*
|
|
PaO2/FiO2
|
59 (39.5, 65.4) |
54 (48.2, 64.1) |
0.917 |
|
Traumatic ARF (%) |
15 (71) |
1 (10) |
0.002*
|
|
Location of ECMO decision (%) |
|
|
0.009*
|
|
Emergency department |
13 (62) |
1 (10) |
|
|
Intensive care unit |
8 (38) |
9 (90) |
|
|
Time interval before ECMO decision, hrs, median (IQR) |
2.2 (1.2, 5.9) |
12.6 (1.7, 44.2) |
0.043*
|
|
CPR (%) |
9 (43) |
1 (10) |
0.106 |
|
ECPR (%) |
1 (5) |
- |
- |
|
Pre-ECMO ventilator duration, days, median (IQR) |
1.0 (1.0, 1.0) |
7.0 (1.0, 16.5) |
0.031*
|
|
ECMO duration, hrs, median (IQR) |
159 (114, 238.5) |
311 (158.3, 599.3) |
0.009*
|
|
Within 24 hrs before and after ECMO |
|
|
|
|
SOFA |
12 (11, 15.5) |
12 (10.8, 15) |
0.755 |
|
SAPS2 |
55 (41.5, 70.5) |
53 (41, 70.5) |
1.000 |
|
Lab findings, mg/dL, median (IQR) |
|
|
|
|
iBUN |
15.9 (10.45, 20.6) |
20. 8 (15.5, 30.3) |
0.065 |
|
iCr |
1.0 (0.75, 1.2) |
1.05 (0.88, 1.35) |
0.633 |
|
pBUN |
31.25 (19.48, 50.03) |
67.95 (40.33, 82.75) |
0.007*
|
|
pCr |
0.95 (0.8, 1.65) |
1.35 (0.88, 1.85) |
0.307 |
|
iTB |
0.88 (0.51, 1.88) |
0.74 (0.29, 1.60) |
0.370 |
|
pTB |
2.43 (1.22, 4.47) |
4.42 (2.59, 13.25) |
0.147 |
|
Lactate, mmol/L |
5.9 (2.45, 9.0) |
3.65 (2.2, 12.3) |
0.698 |
|
Continuous renal replacement therapy (%) |
14 (45) |
7 (23) |
1.000 |
|
Transfusion, units/day, median (IQR) |
|
|
|
|
pRBC |
1.0 (0.7, 2.3) |
1.1 (0.65, 1.23) |
0.519 |
|
FFP |
0.67 (0, 1.25) |
0.5 (0.08, 0.73) |
0.574 |
|
PC |
0.3 (0, 2.72) |
4.2 (0.98, 6.15) |
0.028*
|
|
Hospital days, median (IQR) |
33 (18, 97) |
36 (31, 54) |
0.819 |

Table 3
Clinical Characteristics of the Traumatic and Non-Traumatic Acute Respiratory Failure (ARF) Groups
|
Traumatic ARF (n=16) |
Non-traumatic ARF (n=15) |
p value |
|
Males:females |
14:2 |
11:4 |
0.394 |
|
Age, yrs, median (IQR) |
40 (18, 48) |
53 (41, 69) |
0.006*
|
|
BMI, median (IQR) |
22.5 (20.8, 24.9) |
23.5 (20.8, 25.6) |
0.520 |
|
Pre-ECMO ABGA, median (IQR) |
|
|
|
|
pH |
7.25 (7.05, 7.30) |
7.21 (7.09, 7.34) |
0.953 |
|
PaO2, mm Hg |
59 (43, 64) |
51 (43, 64) |
0.572 |
|
PaCO2, mm Hg |
46 (40, 72) |
59 (53, 82) |
0.110 |
|
PaO2/FiO2
|
59.7 (49.5, 65.9) |
53.8 (46, 64.3) |
0.358 |
|
Location of ECMO decision (%) |
|
|
0.045*
|
|
Emergency department |
10 (63) |
4 (27) |
|
|
Intensive care unit |
6 (38) |
11 (73) |
|
|
Time interval before ECMO decision, hrs, median (IQR) |
1.6 (1.0, 3.4) |
6.1 (2.0, 25.0) |
0.001*
|
|
CPR (%) |
9 (56) |
1 (7) |
0.006*
|
|
ECPR (%) |
1 (6) |
- |
|
|
Pre-ECMO ventilator duration, days, median (IQR) |
1.0 (1.0, 1.0) |
1.0 (1.0, 13.0) |
0.101 |
|
ECMO duration, hrs, median (IQR) |
136 (98.3, 241.5) |
278 (164, 504) |
0.012*
|
|
Within 24 hrs before and after ECMO, median (IQR) |
|
|
|
|
SOFA |
12 (10.3, 14.8) |
12 (11, 15) |
0.682 |
|
SAPS2 |
54 (34, 71.3) |
56 (50, 68) |
0.446 |
|
Lab findings, mg/dL, median (IQR) |
|
|
|
|
iBUN |
14.3 (9.58, 17.53) |
19.3 (15.9, 42.5) |
0.008*
|
|
iCr |
0.95 (0.73, 1.65) |
1.0 (0.9, 1.3) |
0.423 |
|
pBUN |
23.9 (17.28, 51.45) |
57.7 (34.9, 79.8) |
0.017 |
|
pCr |
0.90 (0.73, 1.65) |
1.3 (0.88, 1.85) |
0.179 |
|
iTB |
0.90 (0.65, 1.55) |
0.68 (0.41, 1.86) |
0.495 |
|
pTB |
2.25 (1.59, 4.78) |
3.13 (1.06, 12.46) |
0.446 |
|
Lactate, mmol/L |
6.1 (5.1, 10.1) |
3.2 (1.8, 8.15) |
0.247 |
|
Continuous renal replacement therapy (%) |
9 (29) |
12 (39) |
0.252 |
|
Transfusion, units/day, median (IQR) |
|
|
|
|
pRBC |
0.9 (0.6, 2.1) |
1.2 (0.8, 1.8) |
0.711 |
|
FFP |
0.5 (0, 1.2) |
0.6 (0.1, 0.8) |
0.740 |
|
PC |
0.2 (0, 2.6) |
3.3 (0.6, 5.7) |
0.033*
|
|
Hospital days, median (IQR) |
26.5 (15, 84.5) |
37 (29, 71) |
0.299 |
|
Survival (%) |
15 (94) |
6 (40) |
0.002*
|

Table 4
Complications and Causes of Death of the Patients

|
Complication |
n (%) |
Cause of death |
n (%) |
|
Cannula site bleeding |
1 (3.2) |
Multi-organ failure |
6 (60) |
|
Leg ischemia |
1 (3.2) |
Encephalitis |
1 (10) |
|
Bed sore |
3 (9.7) |
Bronchopleural fistula |
1 (10) |
|
ARF (creatinine >2 mg/dL) |
5 (16.1) |
Give-up |
2 (20) |
|
Cholecystitis |
2 (6.5) |
|
|
|
Ulcer bleeding |
3 (9.7) |
|
|
|
Bronchopleural fistula |
1 (3.2) |
|
|
|
Multiorgan failure |
6 (19.4) |
|
|
|
Cerebral infarction |
1 (3.2) |
|
|
|
Encephalitis |
1 (3.2) |
|
|










 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download