Abstract
Purpose
Ultrasound (US) and US-guided fine needle aspiration biopsies (FNAB) are considered the modalities of choice for assessing lymph nodes suspected of containing metastases, but the sensitivity of FNAB varies and is specific to the operator. We analyzed the risk of FNAB providing false negative results of lateral neck node metastasis, and evaluated diagnostic accuracy of FNAB, in patients with papillary thyroid cancer.
Materials and Methods
FNAB was performed in 242 patients suspected of having lateral neck node metastasis on preoperative imaging. Thyroglobulin in the fine-needle aspirate washout (FNA wash-out Tg) and computed tomography enhancement (Hounsfield units) were measured. Patients with negative results on FNAB were examined by intraoperative frozen section. The false negative and true negative groups were compared.
Results
Of the 242 patients, 130 were confirmed as having lateral neck node metastases. In 74 patients, the metastasis was identified by FNAB. False positive results were observed in 2 patients (0.8%) and false negatives in 58 (44.6%). Risk analysis showed that patient age <45 years (p=0.006), tumor size >1 cm (p=0.008) and elevated FNA wash-out Tg (p=0.004) were significantly associated with false negative results on FNAB. The accuracy of FNAB increased significantly when combined with FNA wash-out Tg (p=0.003).
Papillary thyroid carcinoma (PTC) accounts for about 80% of thyroid carcinomas and usually has a good prognosis, with a mortality rate of less than 10%.1,2 In 30-80% of patients, however, PTC metastasizes to regional lymph nodes.3,4 Many studies have shown prognostic significance of node metastasis in thyroid carcinoma. Furthermore, some groups demonstrated that lateral neck node metastasis is an important prognostic factor for tumor recurrence and poor prognosis,5,6 whereas some opposed.7,8 Precise preoperative detection of lateral neck node metastasis is important in determining the extent of surgery. According to American Thyroid Association (ATA) guidelines, ultrasound-guided fine-needle aspiration biopsy (US-guided FNAB) is the most accurate and cost-effective method for evaluating thyroid nodules and enlarged cervical lymph nodes.9 Despite its usefulness in detecting metastatic lateral neck nodes, US is operator-dependent and is limited in detecting lymph nodes in the retropharyngeal space, the mediastinum, and low level VI.10 Moreover, 5-10% of FNABs are non-diagnostic11 and 6-8% are false negatives.12 Several recent studies have reported that the detection of thyroglobulin (Tg) in fine-needle aspirate washout (FNA wash-out Tg) has a higher sensitivity and specificity in identifying metastatic lymph nodes than FNAB alone.13,14 However, the diagnostic FNA wash-out Tg threshold has not yet been established.13 In contrast to US, computed tomography (CT) is not operator-dependent, and is capable of evaluating whole neck levels. We have analyzed the risk of FNAB providing false negative results of lateral neck node metastasis in patients with PTC, and we also compared the diagnostic accuracy of several preoperative evaluation methods in detecting lateral neck node metastasis in these patients
Between January 1, 2010, and December 31, 2011, 408 patients with PTC suspected of having lateral neck node metastasis on preoperative imaging were operated on by a single surgeon at the Thyroid Cancer Center, Gangnam Severance Hospital, Yonsei University College of Medicine. Of these, 242 consecutive patients underwent US and CT during preoperative evaluation and were enrolled in this study. All patients underwent FNAB. FNA wash-out Tg and CT enhancement [Hounsfield units (HU)] were measured in all suspicious lymph nodes. This retrospective study of medical records was approved by the Institutional Review Board of Yonsei University College of Medicine, Seoul, Korea, which waived requirements for patient approval and informed consent.
US examinations of cervical lymph nodes were performed using a 7- to 15-MHz linear array transducer (HDI 5000; Philips Medical Systems, Bothell, WA, USA) or a 5- to 12-MHz linear array transducer (iU22; Philips Medical Systems). Real-time US was performed by one of four radiologists, all of whom were aware of the patients' clinical his-tories. Suspicious US features of lymph nodes included hy-perechogenicity relative to the surrounding muscles, loss of fatty hilum, cystic changes, calcification, round shape (a long/transverse diameter ratio <1.5), and a chaotic or peripheral vascular pattern.1,10,15 Lymph nodes were considered suspicious when one or more of these suspicious US findings was present.
FNAB of lateral cervical lymph nodes was performed simultaneously with preoperative US staging. US-guided FNAB was performed with a 23-gauge needle attached to a 20-mL disposable plastic syringe and aspirator. Aspirated material was smeared onto glass slides, some of which were immediately immersed in 95% alcohol for Papanicolaou staining. The remaining material was rinsed in saline for cell block processing. FNA wash-out Tg levels were measured by rinsing the same needle and syringe with 1 mL of normal saline.13
All patients underwent contrast-enhanced CT with a multidetector scanner (Somatom Sensation 16 or Somatom Sensation 64; Siemens Healthcare, Erlangen, Germany) with a reconstructed slice thickness of 3 mm for axial and coronal images. A 90-mL dose of iodinated contrast medium (iopromide, Ultravist 300; Bayer Schering Pharma, Berlin-Wedding, Germany) was administered intravenously at a rate of 3 mL/s with an automated injector. A 3-mL/s flush of normal saline solution was injected immediately afterward to reduce artifacts induced in the subclavian vein. The scan delay time was 40-60 s. Suspicious CT features suggesting metastasis included the presence of calcifications, central necrosis or cystic change, and lymph nodes showing heterogeneous cortical enhancement or greater enhancement than the adjacent muscle.1,16 CT enhancement (HU) was considered objectively valuable because it could be quantified. HU was measured by drawing round to oval regions of interest, mostly at the areas of solid contents, showing prominent enhancement in the lymph nodes.
When fine needle aspiration cytology revealed malignant cells in the lymph nodes, unilateral lateral neck dissection was performed along with total thyroidectomy. In patients having lymph nodes with suspicious US or CT features but with no definite metastatic cells on cytology, on-site US-guided localization of lateral neck nodes was performed. Targeting of lateral neck nodes was performed by injecting 0.1 mL of 5% vital dye under US guidance. Utilizing the standard low neck collar incision for thyroidectomy, the stained lateral neck node was excised and sent for frozen-section histologic analysis. The extent of surgery was based on the results of frozen-section examination. If the lymph node was confirmed as metastatic node, lateral neck dissection was performed (Fig. 1). Lateral neck dissection involved removing levels II to IV, or V in some cases. Aspirated lymph nodes were evaluated on a level-by-level basis, and the results of FNAB and pathology were compared
Categorical data were reported as frequencies and percentages, and χ2 tests were used to determine differences between metastatic and benign lymph nodes. Continuous variables were compared using Student's t-tests. Appropriate cut-off values of FNA wash-out Tg and HU in metastatic lateral lymph nodes were calculated by receiver operating characteristic (ROC) analysis. The sensitivity, specificity and accuracy of FNAB, FNA wash-out Tg and HU were calculated on the basis of permanent pathology, and pairwise comparison was determined using area under the curve (AUC). All reported p values are two sided, with p values <0.05 defined as statistically significant. All statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC, USA).
The mean age of the patients was 44.1 years (range, 18-73 years) and the male to female ratio was 1:2.6 (68:174 patients). Of the 242 patients, 130 were confirmed as having metastases. Seventy two patients were confirmed by FNAB and 58 patients were negative results of FNAB but confirmed by intraoperative frozen section (Table 1). Lateral neck dissection involved removing level II to IV or V in some cases. The mean number of dissected lateral lymph nodes was 38 (range, 10-95). The clinicopathologic characteristics of the patients are summarized in Table 2. Patients with lateral neck node metastasis were significantly younger than those without metastasis (p=0.002). The mean tumor size was significantly larger in the metastatic than in the nonmetastatic group (p<0.001). Tumor stage >T 2 (p=0.006), presence of extrathyroidal invasion (p=0.011), and presence of psammomatous calcification (p=0.004) were significantly more frequent in the metastatic group, as were FNA wash-out Tg concentration (p<0.001) and HUs (p=0.009). There were no significant between group differences in gender distribution (p=0.774), presence of multiple tumors (p=0.059), type of tumor margin (p=1.000), and presence of thyroiditis (p=0.787). Multivariate analysis showed that tumor size >1 cm and FNA wash-out Tg >34.8 ng/mL were significantly associated with lateral neck node metastasis (Table 3).
Of the 168 patients with negative results on FNAB, 58 were diagnosed as having lateral neck node metastasis after analysis of intraoperative frozen sections, making the false negative rate of FNAB 44.6%. Clinicopathologic characteristics of the false negative and true negative patients are summarized in Table 4. Patients in the false negative group were significantly younger (p=0.001) and had significantly larger tumors (p=0.002) than patients in the true negative group. Psammomatous calcifications (p=0.020) were significantly more common and FNA wash-out Tg concentration (p=0.004) was significantly higher in the false negative group. Other clinicopathologic features did not differ significantly. Multivariate analysis showed that only a FNA wash-out Tg concentration >34.8 ng/mL was significantly correlated with false negative results on FNAB (p=0.002) (Table 5).
Of the 242 patients, 74 (30.6%) were initially diagnosed with lateral neck node metastasis by FNAB, with 2 (0.8%) being false positives. Moreover, 58 (24.0%) were false negatives. The sensitivity and specificity of FNAB were 55.4% and 98.2%, respectively. ROC analysis was performed on FNA wash-out Tg and HU. Using an FNA wash-out Tg cut-off value of 34.8 ng/mL, AUC was 0.822, the sensitivity was 58.5%, and the specificity was 99.1%. Using an HU cut-off value of 133, the AUC was 0.598 and the sensitivity and specificity were 35.4% and 85.7%, respectively (Table 6). Compared these three methods, we found that the AUCs of FNAB, FNA wash-out Tg, and HU were 0.768, 0.822, and 0.598, respectively, with the AUC of FNA wash-out Tg being significantly higher than that of FNAB (p=0.019), and the AUC of HU being significantly lower than that of FNAB (p<0.001) or FNA wash-out Tg (p<0.001). The accuracy of FNAB increased significantly when combined with FNA wash-out Tg (p=0.003). The AUC increased from 0.768 to 0.829, the sensitivity was 65.4% and the specificity was 97.3%. None of the other pairwise combinations, however, showed significant increase in AUC, sensitivity and specificity (Table 7).
Although preoperative evaluation for lateral neck node metastasis is important in patients with PTC, predicting such metastases is very difficult. Significant clinical factors associated with lateral neck node metastasis include male gender, age <45 years, tumor size >2 cm, and presence of extrathyroidal invasion.17,18 Although observed no significant difference between genders, we found that patient's age, tumor size, and presence of extrathyroidal invasion were significantly associated with lateral neck node metastasis. Moreover, we found that the presence of psammomatous calcification, FNA wash-out Tg, and HU were predictive factors. Multivariate analysis showed that tumor size >1 cm and FNA wash-out Tg >34.8 ng/mL were particularly significant predictive factors.
US and US-guided FNAB are considered the modalities of choice for assessing thyroid nodules and lymph nodes in patients suspected of having thyroid cancer. US has shown variable sensitivity (37-84%), but relatively high specificity (89-98%), in detecting metastatic nodes.1,5 Hyperechogenicity, loss of fatty hilum, cystic change, calcification, round shape, and abnormal vascular pattern are US features associated with metastatic lymph nodes.1,10,15 However, lymph node enlargement and loss of fatty hilum are often seen in normal individuals, making these findings problematic in identifying metastatic lymph nodes.19 Although US and US-guided FNAB are highly accurate, they are operator-dependent; thus diagnoses can vary according to the ability of the operator or pathologist. The false negative rate (44.6%) observed for FNAB, might have been due to aggressive evaluation of suspicious lateral neck nodes, even if FNAB results were negative. Risk analysis of false negativity on FNAB showed that patient age, tumor size, and FNA wash-out Tg were significantly associated with preoperative results, with FNA wash-out Tg concentration being the most significant factor in multivariate analysis. To reduce the false negative rate of FNAB, patient age, tumor size, and FNA wash-out Tg should be considered in preoperative planning.
Tg concentration in FNA washout was initially reported to be useful for early detection of neck lymph node metastases in patients with differentiated PTC.14 This procedure does not require an additional puncture, requires little extra time, and is easy to perform. However, it is very difficult to determine a specific Tg threshold concentration because various kits are used worldwide and also the techniques and volumes used for syringe washout differ considerably. We used an electrochemiluminescence immunoassay, in which the maximum measurable concentration of FNA wash-out Tg level was 5000 ng/mL. FNA wash-out Tg concentration has been reported to be more sensitive than FNAB alone and to increase the sensitivity of FNAB when the two are combined.13 We indeed found that the accuracy of FNA wash-out Tg was significantly higher than that of FNAB (p=0.019), and that the accuracy of FNAB was increased significantly when combined with FNA wash-out Tg (p<0.001).
Although routine preoperative CT has not been recommended by the ATA guidelines,20 CT enhancement (HU) is relatively objective and quantifiable. We found that an HU higher than 110 was significantly associated with the presence of lateral neck node metastasis.4 Although HU was significantly higher in the metastatic group (p=0.009), its sensitivity (35.4%) was lower than that of FNAB or FNA wash-out Tg. Similarly, comparative analysis showed that the accuracy of HU was significantly lower than that of other preoperative methods, and that the accuracy of FNAB was not increased significantly when combined with HU. Elevated HU, nevertheless, may still be useful in diagnosing lateral neck node metastasis.
In conclusion, the false negative rate of FNAB can be reduced by considering patient age (<45 years), tumor size (>1 cm) and FNA wash-out Tg (>34.8 ng/mL) during preoperative planning. The accuracy of FNAB may be improved by combining it with measurements of FNA wash-out Tg.
Figures and Tables
 | Fig. 1Diagram of the algorithm for evaluation and management of lateral neck node. FNAB, fine needle aspiration biopsy; US, ultrasound; FNA, fine needle aspiration; Tg, thyroglobulin; HU, Hounsfield unit. |
Table 1
Relation between Result of FNAB and Final Pathology

| FNAB | Permanent pathology no metastasis (n=112) | Permanent pathology metastasis (n=130) |
|---|---|---|
| Negative (n=168) | 110 (98.2%) | 58 (44.6%) |
| Positive (n=74) | 2 (1.8%) | 72 (55.4%) |
Table 2
Clinicopathologic Characteristics of Patients
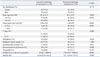
Table 3
Multivariate Analysis of Risk Factors for Lateral Neck Node Metastasis

Table 4
Clinicopathologic Characteristics of Patients with Negative Results on FNAB
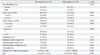
Table 5
Multivariate Analysis of Factors Associated with False Negative Result on FNAB

Table 6
Sensitivity and Specificity of Preoperative Evaluation Methods

| Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC | p value | |
|---|---|---|---|---|---|
| FNAB | 55.4 | 98.2 | 75.2 | 0.768 | <0.001 |
| FNA wash-out Tg (34.8 ng/mL) | 58.5 | 99.1 | 76.9 | 0.822 | 0.002 |
| HU (133) | 35.4 | 85.7 | 58.7 | 0.598 | 0.002 |
Table 7
Comparative Analysis of the Accuracy of Preoperative Evaluation Methods

References
1. Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008; 18:411–418.

2. Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001; 86:1447–1463.

3. Ito Y, Miyauchi A. Lateral lymph node dissection guided by preoperative and intraoperative findings in differentiated thyroid carcinoma. World J Surg. 2008; 32:729–739.

4. Lim CY, Sohn EJ, Lee J, Yun JS, Nam KH, Chang HS, et al. The significant predicting factors influencing lateral neck node metastasis in papillary thyroid carcinoma. J Korean Surg Soc. 2006; 71:326–330.
5. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Ultrasonographically and anatomopathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinoma. World J Surg. 2005; 29:917–920.

6. Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981; 70:511–518.

7. Shaha AR, Shah JP, Loree TR. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann Surg Oncol. 1996; 3:534–538.

8. Steinmüller T, Klupp J, Rayes N, Ulrich F, Jonas S, Gräf KJ, et al. Prognostic factors in patients with differentiated thyroid carcinoma. Eur J Surg. 2000; 166:29–33.

9. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006; 16:109–142.

10. Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol. 2009; 193:871–878.

11. Frasoldati A, Valcavi R. Challenges in neck ultrasonography: lymphadenopathy and parathyroid glands. Endocr Pract. 2004; 10:261–268.

12. Frasoldati A, Toschi E, Zini M, Flora M, Caroggio A, Dotti C, et al. Role of thyroglobulin measurement in fine-needle aspiration biopsies of cervical lymph nodes in patients with differentiated thyroid cancer. Thyroid. 1999; 9:105–111.

13. Kim MJ, Kim EK, Kim BM, Kwak JY, Lee EJ, Park CS, et al. Thyroglobulin measurement in fine-needle aspirate washouts: the criteria for neck node dissection for patients with thyroid cancer. Clin Endocrinol (Oxf). 2009; 70:145–151.

14. Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, et al. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab. 1992; 74:1401–1404.

15. Sohn YM, Kwak JY, Kim EK, Moon HJ, Kim SJ, Kim MJ. Diagnostic approach for evaluation of lymph node metastasis from thyroid cancer using ultrasound and fine-needle aspiration biopsy. AJR Am J Roentgenol. 2010; 194:38–43.

16. Jeong HS, Baek CH, Son YI, Choi JY, Kim HJ, Ko YH, et al. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol (Oxf). 2006; 65:402–407.

17. Jeong JJ, Lee YS, Lee SC, Kang SW, Chung WY, Chang HS, et al. A scoring system for prediction of lateral neck node metastasis from papillary thyroid cancer. J Korean Med Sci. 2011; 26:996–1000.

18. Mirallié E, Sagan C, Hamy A, Paineau J, Kahn X, Le Néel JC, et al. Predictive factors for node involvement in papillary thyroid carcinoma. Univariate and multivariate analyses. Eur J Cancer. 1999; 35:420–423.

19. Yoon JH, Kim JY, Moon HJ, Youk JH, Son EJ, Kim EK, et al. Contribution of computed tomography to ultrasound in predicting lateral lymph node metastasis in patients with papillary thyroid carcinoma. Ann Surg Oncol. 2011; 18:1734–1741.

20. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download