Abstract
Infectious scleritis by Pseudomonas aeruginosa is a well-known vision-threatening disease. In particular, scleral trauma following pterygium surgery may increase the risk of sclera inflammation. Surgical debridement and repair is necessary in patients who do not respond to medical treatments, such as topical and intravenous antibiotics. We reports herein the effectiveness of an autologous perichondrium conchal cartilage graft for infectious scleritis caused by Pseudomonas aeruginosa. This procedure was performed on four eyes of four patients with infectious scleritis who had previously undergone pterygium surgery at Gyeongsang National University Hospital (GNUH), Jinju, Korea from December 2011 to May 2012. Pseudomonas aeruginosa was identified in cultures of necrotic scleral lesion before surgery. The conchal cartilage perichondrium graft was transplanted, and a conjunctival flap was created on the scleral lesion. The autologous perichondrium conchal cartilage graft was successful and visual outcome was stable in all patients, with no reports of graft failure or infection recurrence. In conclusion, autologous perichondrium conchal cartilage graft may be effective in surgical management of Pseudomonal infectious scleritis when non-surgical medical treatment is ineffective. Further studies in larger, diverse populations are warranted to establish the effectiveness of the procedure.
Scleritis is an ocular inflammatory disease with variety of clinical presentations and etiologic factors. Etiologic factors of scleritis vary from idiopathic to autoimmune to infectious.12 However, infectious scleritis is rare, occurring in about 5-10% of all cases, and typically occurs following trauma or surgery, most commonly pterygium surgery.23 As reported previously in India,2 Taiwan3 and western country,4 infectious scleritis had various etiologies, and the most common causative organism of infectious scleritis is Pseudomonas aeruginosa, which has a particularly poor prognosis. Inappropriate treatment of Pseudomonal infectious scleritis may cause severe thinning and melting of the sclera, leading to even eye perforation, and resulting in endophthalmitis and blindness.234
A variety of non-surgical strategies are used for infectious scleritis caused by bacteria, including systemic, topical, and subconjunctival antibiotics, as well as antibiotic-containing wound irrigation solutions.5 However, medical therapy alone is effective only in 18% of cases, as most cases require surgical debridement.4 In the last decade, many tissues and synthetic materials have been used as reconstructive materials, e.g., sclera, cornea, dermis, autologous fascia lata, and Gore-Tex. However, no universally acceptable material has yet been identified. Conchal cartilage has been used in the field of otolaryngology such as septoplasty and ophthalmological procedures such as tarsal reconstruction, and it provided good functional and mechanical integrity in the reconstructed lesion.6 This case report describes the use of autologous perichondrium conchal cartilage graft in four patients with Pseudomonal infectious scleritis.
We described herein four eyes of four patients who underwent autologous perichondrium conchal cartilage grafts from December 2011 to May 2012 at Gyeongsang National University Hospital (GNUH), Jinju, Korea. This study was approved by the GNUH Institutional Review Board and conducted in accordance with the tenets of the Declaration of Helsinki.
Three women (aged 77, 61, and 78 years, respectively) and one man (aged 69 years) were enrolled in our study. None of the patients had systemic disease, and all eyes had a history of pterygium excision surgery approximately 10 years ago in 3 patients and 20 years ago in 61-year-old patient, but no specific previous ocular history otherwise. Before referred to our center, all eyes were treated with topical moxifloxacin 0.5% and tobramycin and without any systemic or topical steroid therapy at local clinics. According to Snellen visual acuity, initial best corrected visual acuity (BCVA) were 20/25, 20/20, 20/25, and 20/63, respectively. Intraocular pressure was in the normal range for all patients. Slit lamp examination showed scleral melting with calcified plaque and subconjunctival abscesses (Table 1).
Before the procedure, patients' scleras were scraped from the base of the active lesion for culture and smears, and then stained with Gram and potassium hydroxide (KOH). In all cases, Gram stain revealed Gram negative rod organisms and negative KOH stains. Initially, moxifloxacin 0.5% (Vigamox®, Alcon, TX, USA) and topical fortified ceftazidime (Tazime®, Hanmi, Seoul, Korea) were applied in every hour, and intravenous ceftazidime (50 mg/mL) were administered twice a day, because of strong suspicion of Pseudomonas infection. However, surgery was ultimately recommended because these non-surgical treatments did not improve the scleral lesions, as shown by scleral thinning and melting along with subconjunctival abscesses. The scleral lesions were cultured before surgery, and Pseudomonas aeruginosa was found in all cultures.
For graft surgery, perichondrium was obtained by otolaryngologists under local anesthesia. The planned area of dissection was marked, and the posterior surface of the auricular cartilage was incised. The dissection was performed down to the perichondrium level and preceded medially above the auricular perichondrium. Auricular cartilage was obtained, leaving the perichondrium attached to the posterior cartilage surface. The incision of subcutaneous tissue and skin was approximated with sutures. The perichondrium was peeled from the cartilage and designed. Ophthalmologic surgery was performed under retrobulbar anesthesia. Necrotized sclera tissues were removed to expose the normal sclera. After defining the borders of the surgical bed to be reinforced, the perichondrium of ear cartilage was fashioned to the appropriate size and thickness. The graft was secured to the edges of the resection site using interrupted 10-0 nylon sutures and covered with a conjunctival flap obtained from the surrounding site by using 10-0 nylon sutures (Fig. 1). On the day after surgery, moxifloxacin 0.5%, topical fortified ceftazidime, and intravenous ceftazidime (50 mg/mL) were administered and then gradually tapered off according to the treatment effect.
No recurrence was noted after surgery in all cases until last follow up (Fig. 2). The final BCVA were 20/20, 20/20, 20/20, and 20/25, respectively.
Although the scleral inflammatory pathways have been investigated in several studies,78 the exact pathogenic mechanisms are not completely clear. However, a disordered immune response leads to both tissue and blood vessel damage, and scleral trauma after surgery may further increase the risk of sclera inflammation and necrosis.49
The epidemiology of infectious scleritis is complex and varies greatly, depending on geographic region.234 In developing countries, fungal etiologies are more common, related to difference in climate.2 However, two large retrospective studies in United States4 and Taiwan3 showed that infectious scleritis caused by bacteria such as Pseudomonas aeruginosa is the most common form in developed countries, accounting for about 85% of all bacterial scleritis cases. Medical management of infectious scleritis includes systemic, topical, and subconjunctival antibiotics, as well as wound irrigation with an antibiotic solution.5 However, most cases require surgical debridement, as only 18% of cases are sufficiently treated with medical therapy alone.4
Many tissues and synthetic materials have been used as reconstructive materials in the last decade,10 including sclera, cornea, dermis, autologous fascia lata, and Gore-Tex, although no universally acceptable materials have been identified. Togo, et al.11 suggested that cells harvested from ear cartilage differentiate into cartilage cells, fat cells, and osteocytes. Thus, the mesodermal stem cells in the ear cartilage perichondrium may be a valuable candidate for surgical management of infectious scleritis due to fast vessel formation and graft re-epithelialization, which promotes successful graft stabilization.
Autologous perichondrium of conchal cartilage grafting had favorable surgical outcomes in our four cases of infectious scleritis caused by Pseudomonas aeruginosa. The structural integrity of the globe was ultimately preserved, the grafts showed good vascularization and epithelialization, visual acuity remained stable, and no graft failures were reported following this management.
In conclusion, autologous conchal cartilage perichondrium grafts maintained functional and structural integrity of the globe and resulted in successful cosmetic and visual outcomes in four cases of infectious scleritis caused by Pseudomonas aeruginosa. Further studies, such as cohort studies, comparative observational studies, and clinical trials, involving a larger number of patients, are warranted to further assess the success of this novel surgical technique for surgical management of Pseudomonal infectious scleritis.
Figures and Tables
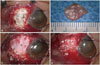 | Fig. 1Intraoperative photographs of the transplantation of perichondrium of conchal cartilage graft. (A) After debridement, necrotic and melted sclera tissue is shown. (B) Perichondrium is harvested from patient's conchal cartilage. (C) The perichondrium graft (asterisk) was transplanted over the sclera defect and sutured with 10-0 nylon sutures. (D) Conjunctival flap over the perichondrium graft is shown. |
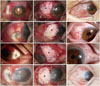 | Fig. 2Photographs of the Pseudomonal infectious scleritis cases. (A, B, and C) Patient 1. (D, E, and F) Patient 2. (G, H, and I) Patient 3. (J, K, and L) Patient 4. (A, D, G, and J) In cases before surgery, severe conjunctival injection and scleral melting with calcified plaque deposits are shown. (B, E, H, and K) Intraoperative photograph of cases shows transplantated perichondrium of conchal cartilage (asterisk) over melting scleral lesion. (C, F, I, and L) After transplantation of perichondrium and conjunctival flap, lesions revealed a structurally and cosmetically stable appearance. |
Table 1
Demographics and Clinical Findings of 4 Patients with Pseudomonal Infectious Scleritis Treated with Autologous Perichodrium Graft of Conchal Cartilage

ACKNOWLEDGEMENTS
This work was supported by Biochemical Research Institute funds (grant no. GNUHBRIF-2014-0003) from the Gyeongsang National University Hospital (Jinju, Korea).
References
1. Shang Y, Han S, Li J, Ren Q, Song F, Chen H. The clinical feature of Behçet's disease in Northeastern China. Yonsei Med J. 2009; 50:630–636.

2. Jain V, Garg P, Sharma S. Microbial scleritis-experience from a developing country. Eye (Lond). 2009; 23:255–261.

3. Ho YF, Yeh LK, Tan HY, Chen HC, Chen YF, Lin HC, et al. Infectious scleritis in Taiwan-a 10-year review in a tertiary-care hospital. Cornea. 2014; 33:838–843.

4. Hodson KL, Galor A, Karp CL, Davis JL, Albini TA, Perez VL, et al. Epidemiology and visual outcomes in patients with infectious scleritis. Cornea. 2013; 32:466–472.

5. Lin CP, Shih MH, Tsai MC. Clinical experiences of infectious scleral ulceration: a complication of pterygium operation. Br J Ophthalmol. 1997; 81:980–983.

6. Parodi PC, Calligaris F, De Biasio F, De Maglio G, Miani F, Zeppieri M. Lower lid reconstruction utilizing auricular conchal chondralperichondral tissue in patients with neoplastic lesions. Biomed Res Int. 2013; 2013:837536.

7. Watson P, Romano A. The impact of new methods of investigation and treatment on the understanding of the pathology of scleral inflammation. Eye (Lond). 2014; 28:915–930.

8. Watson P, Hazleman BL, McCluskey P, Favesio CE. The sclera and systemic disorders. 3rd ed. London: JP Medical Ltd.;2012.
9. Paula JS, Simão ML, Rocha EM, Romão E, Velasco Cruz AA. Atypical pneumococcal scleritis after pterygium excision: case report and literature review. Cornea. 2006; 25:115–117.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download