Abstract
Granular cell tumors (GCTs) are soft tissue tumors, which are thought to be derived from Schwann cells. Although most GCTs are reported to arise in tongue and oral cavity (30-50%), they can appear on any anatomic sites, even visceral organs. Herein, we report 5 cases of GCTs on unusual anatomic locations, such as palm, arm, thigh, finger, and vulvar area. Complete surgical excision is preferred treatment of choice to prevent recurrence. These cases emphasize that GCTs not involving oral cavity are more prevalent than expected, and the diagnosis should be histopathologically confirmed.
Granular cell tumors (GCTs) are uncommon soft tissue tumors, considered to be derived from peripheral nerves, particularly Schwann cells. Although most GCTs are localized in the head and neck regions, they can appear on any anatomic sites, including extremities, genitalia, and even visceral organs.12 Herein, we report 5 cases of GCTs on unusual anatomic locations.
A previously healthy 9-year-old girl presented with a solitary non-tender skin-colored 1×1 cm-sized slightly raised papule on right forearm (Fig. 1A). The presumptive differential diagnosis included dermatofibroma. Punch biopsy showed a dermal tumor composed of nests of large polygonal cells containing abundant eosinophilic cytoplasm with fine granules, consistent with GCT (Fig. 2A). S-100 was positive in tumor cells (Fig. 2B). The tumor was totally excised without recurrence for 4 years.
A 26-year-old female complained of a solitary bean-sized brownish nodule on right palm for 3 years (Fig. 1B). There had been intermittent tenderness, but no difficulties in movement of palm and fingers. Initially, dermatofibroma and schwannoma were clinically suspected. Histopathology demonstrated a well-circumscribed dermal proliferation of cells with small oval nuclei and abundant pink granular cytoplasm arranged in fascicles (Fig. 2C). The tumor cells were stained with S-100 (Fig. 2D). The tumor was surgically removed with no evidence of recurrence for 5 years.
A 69-year-old female patient presented with a linear depression on nail plate with matchhead-sized hyperpigmented crust on right middle fingernail (Fig. 1C). The lesion was intermittently painful. Initially, digital mucous cyst or glomus tumor was suspected. Histopathology of the nail matrix showed proliferation of round cell having abundant eosinophilic granular cytoplasm, suggesting GCT (Fig. 2E). The tumor cells were positive for S-100 (Fig. 2F). The tumor was removed by excision.
A 52-year-old female had a pea-sized slightly brownish palpable firm nodule on the right thigh for several months (Fig. 1D). Clinically, dermatofibroma or calcinosis cutis was considered as differential diagnosis. Punch biopsy revealed sheets and clusters of large cells with abundant granular eosinophilic cytoplasm and small-to-medium sized central round-to-oval nuclei, consistent with GCT (Fig. 2G). Immunohistochemical staining was positive with S-100 in tumor cells (Fig. 2H). The remaining tumor was excised without involvement of resection margin.
Two non-tender nodules, each 3×1.5 cm and 1×1 cm sized, had been on the right labium majora of a 56-year-old female patient for 4 years (Fig. 1E). She had a history of total laparoscopic hysterectomy due to uterine leiomyoma 6 years ago. Histopathologic evaluation showed a circumscribed tumor, composed of large polygonal cells with abundant granular eosinophilic cytoplasm and uniform-looking round to oval nuclei, forming sheets and nests irregularly infiltrating between collagen bundles, consistent with granular cell tumor (Fig. 2I). Both tumors were removed by Mohs micrographic surgery (MMS) and free resection margin was confirmed by frozen section. The cells were positive for S-100 (Fig. 2J) and periodic acid-Schiff (PAS) stain.
GCTs present as asymptomatic small firm skin colored nodules, but pruritus and pain might occasionally be present.3 As GCTs are clinically nonspecific, distinctive histologic findings are essential for diagnosis. GCTs are composed of loosely infiltrating sheets of characteristic large pale polyhedral cells with abundant fine or coarsely granular eosinophilic cytoplasm and a centrally situated pale nucleus.4 Immunohistochemically, GCTs stain positive for S100 protein, and neuron specific enolase, and the granules typically stain with PAS.5 Although most GCTs are considered benign, 1-7% of malignant cases and association with regional or distant metastasis, and multiple organ involvement have been reported.67 Rapid progression, larger size (>4 mm), adjacent tissue invasion, ulceration of the overlying epidermis, cytologic atypia, nuclear pleomorphism, mitotic activity, and necrosis point towards the malignant potential.8 The histological criteria for malignancy are necrosis, spindling, vesicular nuclei with large nucleoli, increased mitotic activity (i.e., more than 2 mitoses per 10 high-power fields at ×200 magnification), high nuclear to cytoplasmic ratio and pleomorphism. Neoplasms that meet three or more of these criteria are considered histologically malignant.9
GCTs preferentially occur on the head and neck region, particularly the tongue and oral cavity (30-50%), the areas with a high density of peripheral nerves.18 Vulvar GCTs are uncommon and have been reported in 5-16% cases.10 The clinical differential diagnoses include fibroma, lipoma, and hidradenoma. Histopathologically, GCTs may be difficult to distinguish from granular cell variants of variable tumors, such as leiomyoma, dermatofibrosarcoma, and angiosarcoma if examined with routine light microscopy alone. Immunohistochemistry helps as GCTs stain negative for desmin, cytokeratins, epithelial membrane antigen and glial fibrillary acidic protein.4 GCTs on the extremities, including palm, thigh, and digits, are even rarer, and a GCT involving fingernail matrix, as seen in our case 3, has never been reported in English literature.
Complete surgical excision is the treatment of choice and, if resection margins are involved, wider local excision may be recommended to decrease the risk of recurrence.11 Some GCTs on extremities might have a far greater tissue depth than clinically suspected, and wider and deeper excision should be considered.2 Our cases 1-4 on extremities were not invading deeper tissue, such as muscle or bone, and simple local excision could successfully remove the entire tumors. For cases with atypical features or malignant potential, wide excision and/or regional lymph node dissection is recommended.12 MMSs are considered when GCTs are malignant or are located in an anatomical region with limited tissue to spare. In case 5, extensive involvement of right labium majora with 2 large nodules made it difficult to spare enough tissue, and MMS was performed.
Reflecting reported female preponderance, all our 5 cases were present in female patients. These cases were located on the anatomic locations not commonly known as predilection sites for GCTs. Because of clinical morphology and the anatomical location, initial clinical differential diagnosis included dermatofibroma, schwannoma for lesions on extremities, and benign cystic lesions for vulvar tumor, but GCTs were not initially considered. These cases emphasize that GCTs not involving oral cavity or tongue are more prevalent than expected, and the diagnosis should be confirmed by histopathology, even when the tumors are clinically suspicious of other tumors.
Figures and Tables
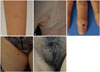 | Fig. 1(A) A solitary skin-colored pea-sized papule on right forearm of a 9-year-old female patient. (B) A bean-sized erythematous to brownish nodule on right palm of a 26-year-old female patient. (C) A longitudinal depression on nail plate with matchhead-sized dark pigmented crust on right third fingernail of a 69-year-old female patient. (D) A pea-sized light brown palpable firm nodule on the right thigh of a 52-year-old female patient. (E) Two non-tender nodules, each 3×1.5 cm and 1×1 cm sized, on the right labium majora of a 56-year-old female patient. |
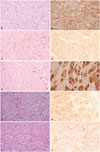 | Fig. 2Microphotographs of the tumors. (A) In case 1, a dermal tumor composed of fascicles, nests of large eosinophilic polygonal cells with granular cytoplasm (H&E, ×400). (B) Tumor cells were positive for S-100 immunostain (S-100, ×400). (C) The tumor cells of case 2 contain abundant eosinophilic cytoplasm with fine granules, suggesting granular cell tumor (H&E, ×400). (D) Immunhistochemical stain with S-100 was positive in tumor cells (S-100, ×400). (E) Nail matrix biopsy of the case 3 revealed proliferations of round cells with abundant eosinophilic cytoplasm (H&E, ×400). (F) Immunhistochemical stain with S-100 was positive in tumor cells (S-100, ×400). (G) Histopathologic evaluation of the tumor in case 4 shows tumor cells with eosinophilic granular cytoplasm (H&E, ×400). (H) S-100 was positively stained in tumor cells (S-100, ×400). Microphotographs of the tumors. (I) Infiltrating sheets of tumor cells with eosinophilic granular cytoplasm, consistent with granular cell tumor (H&E, ×400). (J) The cells were positive for S-100 staining (S-100, ×400). |
References
1. Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980; 13:301–316.

2. Newman E, Eichenfield LF. Granular cell tumor on the palm of an 8-year-old girl. Pediatr Dermatol. 2010; 27:656–657.

3. de Misa RF, Castro V, Suárez J, Perera A. Pruritic vulvar nodule in a black woman. Diagnosis: Granular cell tumor (Abrikossoff tumor). Arch Dermatol. 2000; 136:1165–1170.
4. Hong SC, Lim YK, Chew SH, Chia YN, Yam KL. Case report of granular cell tumor of the vulva and review of current literature. Gynecol Oncol Case Rep. 2012; 3:20–22.

5. Rosai J, Ackerman LV. Rosai and Ackerman's surgical pathology. 9th ed. Edinburgh: Mosby;2004.
6. Apisarnthanarax P. Granular cell tumor. An analysis of 16 cases and review of the literature. J Am Acad Dermatol. 1981; 5:171–182.
7. Ramos PC, Kapp DS, Longacre TA, Teng NN. Malignant granular cell tumor of the vulva in a 17-year-old: case report and literature review. Int J Gynecol Cancer. 2000; 10:429–434.

8. Torrijos-Aguilar A, Alegre-de Miquel V, Pitarch-Bort G, Mercader-García P, Fortea-Baixauli JM. [Cutaneous granular cell tumor: a clinical and pathologic analysis of 34 cases]. Actas Dermosifiliogr. 2009; 100:126–132.

9. Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998; 22:779–794.
10. Cheewakriangkrai C, Sharma S, Deeb G, Lele S. A rare female genital tract tumor: benign granular cell tumor of vulva: case report and review of the literature. Gynecol Oncol. 2005; 97:656–658.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download