Abstract
Purpose
The purpose of our study was to investigate the effect of gait training with rhythmic auditory stimulation (RAS) on both kinematic and temporospatial gait patterns in patients with hemiplegia.
Materials and Methods
Eighteen hemiplegic patients diagnosed with either cerebral palsy or stroke participated in this study. All participants underwent the 4-week gait training with RAS. The treatment was performed for 30 minutes per each session, three sessions per week. RAS was provided with rhythmic beats using a chord progression on a keyboard. Kinematic and temporospatial data were collected and analyzed using a three-dimensional motion analysis system.
Results
Gait training with RAS significantly improved both proximal and distal joint kinematic patterns in hip adduction, knee flexion, and ankle plantar flexion, enhancing the gait deviation index (GDI) as well as ameliorating temporal asymmetry of the stance and swing phases in patients with hemiplegia. Stroke patients with previous walking experience demonstrated significant kinematic improvement in knee flexion in mid-swing and ankle dorsiflexion in terminal stance. Among stroke patients, subacute patients showed a significantly increased GDI score compared with chronic patients. In addition, household ambulators showed a significant effect on reducing anterior tilt of the pelvis with an enhanced GDI score, while community ambulators significantly increased knee flexion in mid-swing phase and ankle dorsiflexion in terminal stance phase.
Conclusion
Gait training with RAS has beneficial effects on both kinematic and temporospatial patterns in patients with hemiplegia, providing not only clinical implications of locomotor rehabilitation with goal-oriented external feedback using RAS but also differential effects according to ambulatory function.
Pathological gait patterns are generally observed in patients with hemiplegia caused by stroke, cerebral palsy (CP), or traumatic brain injury.123 Hemiplegic patients exhibit heterogeneous gait impairments in both the affected and unaffected lower extremities, resulting in abnormal kinematic characteristics and temporospatial asymmetry during their gait.456 Common pathological gait patterns exhibited by hemiplegic patients are abnormal proximal joint movement, such as excessive anterior tilt of the pelvis, as well as abnormal distal joint movement, including foot drop during gait.78910 In addition, these abnormal kinematic patterns lead to decreased cadence and walking velocity and an unbalanced stance and swing phase, eventually reducing energy efficiency.111213 Due to these gait deficiencies, patients with hemiplegia experience serious barriers to functional recovery, as locomotive ability is essential for many daily activities.
Several therapeutic interventions, including conventional treadmill training, body weight-supported treadmill training, robot-assisted gait training, and hippotherapy, are components of rehabilitation for patients with hemiplegia.14151617 As a specific gait-training intervention has not yet been deemed superior by a sufficient amount of evidence, the clinical issue of gait improvement has continually drawn considerable attention from clinicians and researchers.18 Recently, one promising gait training method developed to improve gait impairment is rhythmic auditory stimulation (RAS), which has been applied for a variety of neurological diseases including stroke, CP, Parkinson's disease, traumatic brain injury, and spinal cord injury.1920212223242526272829 Gait training with RAS emphasizes rhythmic bilateral movements providing rhythmic cueing using music elements such as a tempo and beats with chords to ameliorate asymmetry, which is an unrelenting problem in patients with hemiplegia.30 The basic mechanism of gait training with RAS is to regulate repeated movements by auditory-motor synchronization in the central nervous system. An auditory-motor synchronization mechanism is organized isochronously by neural substrates and reflects auditory rhythm and tempo in functional motor output, such as a gait pattern (i.e., velocity, cadence, and stride length in a given period).31 RAS is based on an entrainment model in which rhythmic auditory cues synchronize motor responses into a stable time relationship. In other words, rhythm serves as an anticipatory and continuous time reference on which functional movements are paced or mapped within a stable temporal template. Therefore, entrainment between auditory stimulation and motor responses makes gait pattern regulated and stable in patients with gait deficit.19 Based on the results of previous studies, external auditory cues may rhythmically stimulate neural circuits entraining subcortical systems and lead to the optimization of motor commands.19313233
Most previous studies regarding gait training with RAS in patients with neurological impairments have demonstrated improvement in temporospatial gait parameters, including cadence, walking velocity, and stride length.19212230323435 Thaut, et al.1932 showed that RAS significantly improved walking velocity, stride length, cadence, and symmetry in acute hemiplegic patients who had suffered from stroke. Suh, et al.35 found that three-week gait training with RAS had a significant effect on the gait parameters of walking velocity, stride length, and cadence as well as standing balance in hemiplegic patients following stroke. Hashiguchi, et al.36 reported that RAS resulted in significant gait improvement by increasing gait velocity while simultaneously decreasing the gait variability of stride time in subacute hemiplegic patients after stroke.
In patients with CP who have bilateral involvement, recent studies on gait training with RAS revealed that improvements of both kinematic and temporospatial gait parameters can benefit patients with gait impairments.2122 Kim, et al.2122 showed that significant improvement in proximal limb movements was observed after immediate and long-term gait training with RAS in patients with CP. Thus, statistical significance in ameliorating the anterior tilt of the pelvis and hip flexion has been identified in patients with bilateral spastic CP.
Throughout these previous studies, gait training with RAS was shown to establish a promising therapeutic purpose in the rehabilitation of gait disturbances. However, there is little evidence indicating that RAS treatment for hemiplegic gait patterns pertains to three-planar analysis in the sagittal, coronal, and transverse planes of lower extremity joint kinematics, such as those of the pelvis, hip, knee, ankle, and foot. Therefore, it remains an important challenge to refine the effects of gait training with RAS in order to confirm the changes of both kinematic and temporospatial characteristics in patients with hemiplegia and to compare the kinematic changes according to the etiology of the neurological disorder, onset duration, and ambulatory status.
Eighteen individuals with hemiplegia who were diagnosed with either CP or stroke were recruited in this study. The procedure was approved by the Institutional Review Board (4-2012-0483). Informed consent was obtained from all participants after the experimental procedures were sufficiently explained and before the study began. The inclusion criteria for individuals with hemiplegia were as follows: each participant 1) had no discernible hearing deficit, 2) was able to walk independently for a distance of at least 10 m without the use of a walking aid or supporter, and 3) was able to understand the command to walk following RAS.22
Demographical and clinical characteristics including age, gender, height, weight, body mass index, diagnosis, and ambulatory status are shown in Table 1. Participants were classified as either community ambulators or household ambulators according to their ambulatory status. Community ambulators were able to independently walk around on level ground, curbs, and uneven terrain outdoors as well as indoors for a minimum of 150 feet; they were also able to manage stairs and challenging community activities, such as making visits in their neighborhood. Conversely, household ambulators were only able to walk indoors for short distances (a maximum of 50 feet) either independently or with orthopedic devices; they also encountered difficulty with stairs and uneven terrain and required assistance or walking aids when leaving the house.2137
All participants underwent the 4-week gait training with RAS on the carpet of the same clinic. The RAS treatment was performed for 30 minutes per each session, three sessions per week. The intervention procedure consisted of the following established protocol:2122 1) A participant independently walked barefoot along a 10-m flat walkway three times without RAS at the individual's preferred walking speed. 2) Walking cadence (steps/min) was calculated based on the gait parameters outlined in step 1. 3) The identified initial tempo signaled by metronome beats (beats per minute) was set to the participant's cadence obtained in step 2 in each session. 4) RAS was then provided by the music therapists, who played a live rhythmic pattern using a composed four-chord progression with metronome beats on a keyboard (PSR-E213, Yamaha Electronics Co., Hamamatsu, Japan). To ensure accuracy and consistency of the rhythmic stimulus, the same therapists performed the procedure for each patient. These specialized therapists provided a regular and stable rhythm. To avoid rhythmic monotonousness, several chord progressions were applied. 5) The same chord pattern was repeated to provide a continuous timing cue and a period of 2 minutes to help each participant quickly adapt to the RAS. In this step, the music therapist instructed each participant to finger-tap the rhythm for 1 minute to ensure that they could hear the sounds and also confirmed that each participant could walk comfortably by allowing them to adapt to the RAS for 1 minute. 6) Each participant then walked the length of 10 m three to six times with RAS and rested for 1-3 minutes between walks, depending on the endurance level of the patient. This step was repeated 5-8 times in a session. To monitor compliance of the participants to the RAS, three music therapists evaluated the change in walking speed.2122 7) The final 1-2 minutes was spent by fading out the rhythmic stimulation to monitor the independent carryover effect, which was qu-antified by calculating cadence. These seven steps were applied in each training session during a period of 4 weeks.
All participants were pre- and post-tested 2 days before starting and 2 days after conducting the 4-week gait training with RAS in the gait analysis laboratory. Kinematic and temporospatial data from the pelvis, hip, knee, ankle, and foot were collected and analyzed for the gait trials without RAS. To ensure that reflective foot markers could be recognized from the infrared signal of the motion analysis system, participants walked barefoot along a 10-m flat walkway three times at the individual's preferred walking speed without RAS. For kinematic analysis, 15 passively reflective markers were adhered with specialized tape to the sacrum, both sides of the anterior superior iliac spine, middle thigh, lateral knee, middle shank of the tibia, lateral malleolus, heel, and forefoot.22 A three-dimensional motion analysis system with six cameras (Vicon Nexus ver. 1.8.5, Vicon Motion System Ltd., Oxford, UK) was used to record kinematics by measuring the degree of joint motion during gait performance. This system comprised six infrared-sensitive cam-eras for locating and tracking the fixed retro-reflective markers through space.
The motion analysis system was calibrated before each gait analysis. Participants were simultaneously videotaped from the front and side, and measurements were recorded in the sa-gittal, coronal, and transverse planes. Kinematic data included the angle of pelvic tilt, pelvic obliquity, pelvic rotation, hip flexion and extension, hip adduction and abduction, hip internal and external rotation, knee flexion and extension, ankle plantar and dorsiflexion, and foot internal and external rotation. All kinematic and temporospatial data were processed and plotted, and the graphs were visualized using Polygon software ver. 3.5.1 (Oxford Metrics Inc., Oxford, UK), which was interworked with a three-dimensional motion analysis system. To perform the statistical analysis, three points from each joint range of motion from continuous raw data in the lower extremity were used: initial contact (the moment when the heel struck the floor), the minimal joint angle, and the maximal joint angle during the whole gait cycle.22
In order to evaluate the overall gait pathology, a global index of three-dimensional kinematic changes, the gait deviation index (GDI),38 was utilized in the pre- and post-RAS treatment. The GDI is a scaled distance between nine individual kinematic variables of pathological gait (pelvic tilt, pelvic obliquity, pelvic rotation, hip flexion, hip adduction and abduction, hip internal and external rotation, sagittal angles of the knee and ankle, and foot progression in the transverse plane) and the average of kinematic variables of the reference normal gait. One stride obtained from each pre- and post-treatment gait analysis was selected from the hemiplegic side of every participant. All kine-matic variables were extracted and sampled at every 2% inter-val in the whole gait cycle. Each set of 459 data points (9 joint angles×51 points) was computed as gait vectors using the gait feature of a normal gait referred to as orthonormal f-basis. The computing method is explained in detail in another referenced paper.38 The GDI score can be interpreted as follows: a score of 100 indicates a GDI equal to the average of the normal control. In our study, the GDI was utilized to ensure that individual pathological gait improved after gait training with RAS compared to the normal reference. The GDI score was calculated to take into account the overall effect of kinematic changes of the pelvis, hip, knee, ankle, and foot throughout the gait cycle. In other words, the GDI incorporated the pelvic tilt, pelvic obliquity, pelvic rotation, hip flexion, hip adduction and abduction, hip internal and external rotation, the sagittal angles of the knee and ankle, and the foot progression in the transverse plane.
Temporospatial parameters such as cadence (steps/min), walking velocity (m/sec), stride length (m), step length (m), stride time (sec), step time (sec), single limb support (%), double limb support (%), stance phase (%), and swing phase (%) were calculated using Polygon software ver. 3.5.1 (Oxford Metrics Inc., Oxford, UK). To evaluate the side-to-side asymmetry between the lower limbs, the absolute difference between the unaffected and affected sides was analyzed using temporospatial data.22
All statistical analyses were performed with SPSS Statistics 20 (IBM Corp., Armonk, NY, USA). Wilcoxon signed-rank tests were used to evaluate intra-subject pairwise comparisons between gait parameters: kinematics and temporospatial data and the GDI score in the pre- and post-RAS treatment. Mann-Whitney U tests were also used to compare the inter-group designs of general characteristics and gait parameters. All statistical significance levels were set at p<0.05.
Among total 18 hemiplegic patients, seven subjects diagnosed with CP and eleven subjects diagnosed with stroke participated in this study. Nine subjects with community ambulatory function and nine subjects with household ambulatory function participated. All participants showed mild spasticity of the lower extremities with a grade of 1 to 1+ on the Modified Ashworth Scale. The onset duration of stroke was 3.58±2.22 years. Detailed information on gender, age, height, weight, and body mass index is shown in Table 1. Considering that CP is a developmental disorder that affects the initial acquisition of gait while stroke patients have previous walking experience, these two neurologic disorders represented in the study population were separately analyzed. There were no significant differences in the parameters of general characteristics between CP and stroke (Table 1). In addition, there were no significant differences in the general parameters between community ambulators and household ambulators (Table 1).
When kinematic characteristics of the hemiplegic side were evaluated before and after RAS treatment, pelvic anterior tilt in the sagittal plane largely tended to decrease after gait training with RAS. Hip adduction significantly increased in the mid-stance phase after the RAS treatment (p=0.039 by Wilcoxon signed-rank test) (Table 2). Maximal knee flexion increased in the mid-swing phase (p=0.022), and ankle plantar flexion decreased at initial contact (p=0.025) and push off (p=0.006), while ankle dorsiflexion increased in the terminal stance (p=0.031). When a comprehensive measure of overall gait pathology, GDI, was calculated, the GDI score was found to significantly improve in patients with hemiplegia after RAS treatment (83.89±2.72 to 87.29±2.65, p=0.043) (Table 2).
When temporospatial parameters on the hemiplegic side were evaluated both before and after RAS treatment, there were no significant changes in the statistical analysis after gait training with RAS (Table 3). However, when the side-to-side differences between the unaffected and the hemiplegic side of the temporospatial parameters were evaluated, the side-to-side difference of the stance phase (6.95±0.99% to 4.62±1.15%; p=0.006 by Wilcoxon signed-rank test) and the swing phase (12.12±5.06% to 4.62±1.15%; p=0.006) significantly decreased after the RAS treatment (Table 3), suggesting that gait training with RAS could improve the side-to-side symmetry in patients with hemiplegia.
We additionally analyzed the kinematic data of CP and stroke separately. Stroke patients showed significant kinematic improvement in maximal knee flexion in mid-swing phase (48.88±4.31 to 55.31±3.90; p=0.021 by Wilcoxon signed-rank test) and in maximal ankle dorsiflexion in terminal stance (13.79±1.27 to 16.15±1.42; p=0.026) (Table 4). Patients with CP, on the other hand, did not show kinematic improvement after gait training with RAS (Table 4). This result suggests that the previous walking experience in stroke patients who have a history of typical gait may have an effect on their response to RAS.
Based on longitudinal studies suggesting that motor recovery reaches a plateau until the first 6 months post-stroke,39 we additionally analyzed the effects of RAS on kinematic patterns in both subacute and chronic stroke patients separately. Although chronic stroke patients did not show an increase in GDI score, these patients did show improvement in hip external rotation (0.26±3.57 to -3.98±3.13; p=0.028), maximal ankle dorsiflexion at terminal stance (14.08±1.98 to 16.85±1.72; p=0.028), and foot external rotation (-6.23±2.47 to -9.69±1.44; p=0.028) at initial contact after gait training with RAS (Table 5). On the other hand, subacute stroke patients showed a significant increase in GDI score (80.88±3.82 to 88.99±5.00; p=0.043 by Wilcoxon signed-rank test) with improvement in maximal knee flexion in mid-swing phase (45.15±3.59 to 56.42±4.74; p=0.043) (Table 5). This result suggests that subacute stroke patients are more likely to respond to RAS than chronic patients.
In the analysis of kinematic characteristics, household ambulators showed that pelvic anterior tilt at initial contact (10.74±2.28 to 7.93±1.97; p=0.038 by Wilcoxon signed-rank test) and the minimal angle of anterior tilt (10.19±2.40 to 7.43±1.99; p=0.038) were ameliorated after gait training with RAS. On the other hand, community ambulators were found to have significant increases in maximal knee flexion in the mid-swing phase (60.79±4.67 to 63.82±4.40; p=0.021) and ankle dorsiflexion in the terminal stance (13.86±2.42 to 16.97±2.12; p=0.008), whereas ankle plantar flexion at push-off decreased (-9.39±2.20 to -6.92±2.06; p=0.021) (Table 6). In the analysis of GDI score, household ambulators, and not community ambulators, show-ed significant gait improvement (80.07±3.36 to 86.32±3.82; p=0.008 by Wilcoxon singed-rank test) (Table 6). The kinematic changes of the proximal pelvic joint and distal ankle joint at pre- and post-RAS treatment showed differential patterns according to the ambulatory status (Fig. 1).
Hemiplegic gait pattern, which is observed in patients with stroke or CP, is characterized by laborious and imbalanced limb movement, as an asymmetrical kinematic and temporospatial pattern occurs during locomotion. In addition, patients with hemiplegia have difficulties maintaining shock absorption and weight acceptance in the stance phase as well as accelerating forward propulsion with adequate limb excursion in the swing phase.40
CP is a developmental disorder that affects the initial acquisition of gait, while stroke patients do have a history of typical gait. Previous walking experience in stroke patients may affect their response to RAS, as evidenced by significant kinematic improvement in maximal knee flexion in mid-swing and maximal ankle dorsiflexion in terminal stance. Nevertheless, when kinematic and temporospatial data were evaluated to compare inter-group design using the Mann-Whitney U test, there were no significant differences between CP and stroke patients. Therefore, all data from CP and stroke participants were integrated with hemiplegic patients to present the effect of gait training with RAS in this study. Overall, gait training with RAS significantly improved the GDI score, demonstrating the benefits of RAS treatment on the overall kinematic gait patterns in patients with hemiplegia. Additionally, subacute stroke patients were shown to have significant increases in GDI score, suggesting that subacute patients are more likely to respond to RAS than chronic patients.
In the analysis of proximal joint motion, excessive anterior tilt of the pelvis was significantly reduced in household ambulators. In concordance with previous studies of CP patients with spastic diplegia,2122 this finding supports the hypothesis that gait training with RAS also alleviates excessive anterior tilt of the pelvis in hemiplegic patients with stroke or CP who have household ambulatory function. Abnormal pelvic control is a major problem that can lead to a distorted gait pattern due to the linkage of distal joint movement.41 Hip adduction in the mid-stance phase was also ameliorated in patients with hemiplegia after gait training with RAS. Hemiplegic patients commonly show less hip adduction than healthy controls in the stance phase. As this coronal kinematic deviation reflects poor stability in both the stance and swing phase during gait,42 RAS treatment eventually improves walking stability.
In the analysis of distal joint motion, knee flexion in the mid-swing phase increased in patients with hemiplegia, particularly in community ambulators. This finding suggests that increased knee flexion may reduce toe dragging and compensative pelvic hiking, inducing relatively adequate limb propulsion during the swing phase of gait.43 Ankle dorsiflexion in the terminal stance phase also increased in community ambulators, demonstrating that reduced ankle plantar flexion at initial contact sequentially exhibited increased ankle dorsiflexion in the terminal stance. In other words, the first and second ankle rockers were relatively normalized after gait training with RAS, intuitively suggesting increased stance stability of the hemiplegic side.2144
The potential therapeutic rationale for such differential kinematic effects of RAS according to ambulatory status may involve the proximal-to-distal functional relationship.45 As community ambulators perform relatively adequate proximal mo-tor function, rhythmic auditory cues might be sufficient to facilitate distal joint movement during gait. However, in house-hold ambulators, such cues might be prerequisite to facilitate proximal joint movement in order to follow the RAS during gait training.
In the analysis of overall gait pathology using the GDI, gait impairments improved in patients with hemiplegia. It may be possible that community ambulators showed less improved GDI scores, as the proximal joint movements were less susceptible to the RAS than distal joint movement, representing a ceiling effect of proximal function.
Patients with hemiplegia have been reported to exhibit bilateral differences, showing a reduced stance phase and single limb support of the affected side yet an increased stance phase of the less affected side and double limb support during gait.46 The bilateral difference markedly affects temporospatial asymmetry, including cadence, walking velocity, step length, and stride length. Therefore, an increase of bilateral symmetry indicates motor recovery, which promotes a reciprocal gait pattern.32 As expected from previous studies regarding RAS,2130 the present study revealed that repetitive and rhythmic auditory cues are efficient for hemiplegic patients to achieve bilateral symmetry of the lower extremities during gait. When temporospatial parameters were evaluated in this study, the side-to-side asymmetry of the stance and swing phases was improved in patients with hemiplegia.
The results of this study indicate that gait training with RAS can augment the therapeutic advantages of the proximal kinematic patterns in patients with hemiplegia who are household ambulators. Hence, this study invites additional investigation that would involve more intensive or long-term intervention to confirm the clinical insight of rehabilitation in patients with hemiplegia who are community ambulators. One limitation of this study was that all participants had received conventional physical therapy in the past and during the course of the study. Therefore, further study of a randomized controlled trial is necessary to compare the RAS treatment effects with those of a conventional physical therapy group. This study also needs future investigation to better suggest a neural control mechanism to entrain isochronic-rhythmic stimulation and motor responses, such as gait, as previous studies have not shown a clear mechanism of RAS.303133
In conclusion, as the first clinical trial to investigate the effect of gait training with RAS on both kinematic and temporospatial changes in patients with hemiplegia, this study provided not only clinical implications for locomotor rehabilitation with goal-oriented external feedback using RAS but also differential effects according to ambulatory function.
Figures and Tables
 | Fig. 1The kinematic changes of proximal pelvic joint and distal ankle joint at pre- and post-RAS treatment. According to the ambulatory status, kinematic analysis showed differential patterns after gait training with RAS in patients with hemiplegia. Especially, community ambulators showed proximal pelvic improvement (A and B), while household ambulators showed distal ankle joint improvement (C and D). Dotted line: pre-treatment; black line: post-treatment; gray line: normal range. RAS, rhythmic auditory stimulation. |
Table 1
General Characteristics of Participants

Table 2
Changes in Kinematic Patterns of Hemiplegic Limbs after Gait Training with RAS
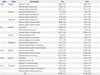
Table 3
Changes in Temporospatial Parameters of Hemiplegic Limbs after Gait Training with RAS
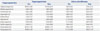
Table 4
Comparison of Kinematic Patterns after Gait Training with RAS According to the Etiology of Neurologic Disorders

Table 5
Comparison of Kinematic Patterns after Gait Training with RAS According to Onset Duration in Stroke Patients

Table 6
Comparison of Kinematic Patterns after Gait Training with RAS According to Ambulatory Status

ACKNOWLEDGEMENTS
This research was supported by the R&D grant (No. 2015007) on rehabilitation from the Korea National Rehabilitation Center Research Institute, Ministry of Health & Welfare.
References
1. Galli M, Cimolin V, Rigoldi C, Tenore N, Albertini G. Gait patterns in hemiplegic children with Cerebral Palsy: comparison of right and left hemiplegia. Res Dev Disabil. 2010; 31:1340–1345.

2. Hillier SL, Sharpe MH, Metzer J. Outcomes 5 years post-traumatic brain injury (with further reference to neurophysical impairment and disability). Brain Inj. 1997; 11:661–675.
3. Rodda J, Graham HK. Classification of gait patterns in spastic hemiplegia and spastic diplegia: a basis for a management algorithm. Eur J Neurol. 2001; 8:Suppl 5. 98–108.

4. Böhm H, Döderlein L. Gait asymmetries in children with cerebral palsy: do they deteriorate with running? Gait Posture. 2012; 35:322–327.

5. Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003; 84:1185–1193.

6. Patterson KK, Parafianowicz I, Danells CJ, Closson V, Verrier MC, Staines WR, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. 2008; 89:304–310.

7. Salazar-Torres JJ, McDowell BC, Kerr C, Cosgrove AP. Pelvic kinematics and their relationship to gait type in hemiplegic cerebral palsy. Gait Posture. 2011; 33:620–624.

8. Chen CL, Chen HC, Tang SF, Wu CY, Cheng PT, Hong WH. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003; 82:925–935.

9. Voigt M, Sinkjaer T. Kinematic and kinetic analysis of the walking pattern in hemiplegic patients with foot-drop using a peroneal nerve stimulator. Clin Biomech (Bristol, Avon). 2000; 15:340–351.

10. Embrey DG, Holtz SL, Alon G, Brandsma BA, McCoy SW. Functional electrical stimulation to dorsiflexors and plantar flexors during gait to improve walking in adults with chronic hemiplegia. Arch Phys Med Rehabil. 2010; 91:687–696.

11. Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture. 2009; 29:129–137.

12. Roerdink M, Beek PJ. Understanding inconsistent step-length asymmetries across hemiplegic stroke patients: impairments and compensatory gait. Neurorehabil Neural Repair. 2011; 25:253–258.

13. Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007; 88:43–49.

14. Pohl M, Mehrholz J, Ritschel C, Rückriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke. 2002; 33:553–558.

15. Yang YR, Chen IH, Liao KK, Huang CC, Wang RY. Cortical reorganization induced by body weight-supported treadmill training in patients with hemiparesis of different stroke durations. Arch Phys Med Rehabil. 2010; 91:513–518.

16. Patritti BL, Straudi S, Deming LC, Benedetti MG, Nimec DL, Bonato P. Robotic gait training in an adult with cerebral palsy: a case report. PM R. 2010; 2:71–75.

17. Park ES, Rha DW, Shin JS, Kim S, Jung S. Effects of hippotherapy on gross motor function and functional performance of children with cerebral palsy. Yonsei Med J. 2014; 55:1736–1742.

19. Thaut MH, Leins AK, Rice RR, Argstatter H, Kenyon GP, McIntosh GC, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil Neural Repair. 2007; 21:455–459.

20. Cha Y, Kim Y, Chung Y. Immediate effects of rhythmic auditory stimulation with tempo changes on gait in stroke patients. J Phys Ther Sci. 2014; 26:479–482.

21. Kim SJ, Kwak EE, Park ES, Lee DS, Kim KJ, Song JE, et al. Changes in gait patterns with rhythmic auditory stimulation in adults with cerebral palsy. NeuroRehabilitation. 2011; 29:233–241.

22. Kim SJ, Kwak EE, Park ES, Cho SR. Differential effects of rhythmic auditory stimulation and neurodevelopmental treatment/Bobath on gait patterns in adults with cerebral palsy: a randomized controlled trial. Clin Rehabil. 2012; 26:904–914.

23. Arias P, Cudeiro J. Effects of rhythmic sensory stimulation (auditory, visual) on gait in Parkinson's disease patients. Exp Brain Res. 2008; 186:589–601.

24. Arias P, Cudeiro J. Effect of rhythmic auditory stimulation on gait in Parkinsonian patients with and without freezing of gait. PLoS One. 2010; 5:e9675.

25. Kadivar Z, Corcos DM, Foto J, Hondzinski JM. Effect of step training and rhythmic auditory stimulation on functional performance in Parkinson patients. Neurorehabil Neural Repair. 2011; 25:626–635.

26. Thaut MH, Gardiner JC, Holmberg D, Horwitz J, Kent L, Andrews G, et al. Neurologic music therapy improves executive function and emotional adjustment in traumatic brain injury rehabilitation. Ann N Y Acad Sci. 2009; 1169:406–416.

27. de l'Etoile SK. The effect of rhythmic auditory stimulation on the gait parameters of patients with incomplete spinal cord injury: an exploratory pilot study. Int J Rehabil Res. 2008; 31:155–157.
28. Kim SJ, Cho SR, Oh SJ, Kwak EE. Case Study of Gait Training Using Rhythmic Auditory Stimulation (RAS) for a Pediatric Patient with Cerebellar Astrocytomas. J Music Hum Behav. 2010; 7:65–81.
29. Han SJ, Kwon AJ, Park HY. Immediate Effect of Patterned Sensory Enhancement (PSE) on Upper Limb Function after Stroke. J Music Human Behav. 2014; 11:1–19.
30. Kwak EE. Effect of rhythmic auditory stimulation on gait performance in children with spastic cerebral palsy. J Music Ther. 2007; 44:198–216.

31. Thaut MH. Neural basis of rhythmic timing networks in the human brain. Ann N Y Acad Sci. 2003; 999:364–373.

32. Thaut MH, McIntosh GC, Rice RR. Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J Neurol Sci. 1997; 151:207–212.

33. McIntosh GC, Brown SH, Rice RR, Thaut MH. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997; 62:22–26.

34. Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N. Rhythmic auditory stimulation modulates gait variability in Parkinson's disease. Eur J Neurosci. 2007; 26:2369–2375.

35. Suh JH, Han SJ, Jeon SY, Kim HJ, Lee JE, Yoon TS, et al. Effect of rhythmic auditory stimulation on gait and balance in hemiplegic stroke patients. NeuroRehabilitation. 2014; 34:193–199.

36. Hashiguchi Y, Ohata K, Kitatani R, Sakuma K, Watanabe A, Yamakami N. Effect of rhythmic auditory stimulation on gait parameters and gait emg in patients with hemiplegia after stroke more less. Gait Posture. 2014; 39:S139.
37. Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995; 26:982–989.

38. Schwartz MH, Rozumalski A. The Gait Deviation Index: a new comprehensive index of gait pathology. Gait Posture. 2008; 28:351–357.

39. Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006; 19:84–90.

41. Lennon S. Gait re-education based on the Bobath concept in two patients with hemiplegia following stroke. Phys Ther. 2001; 81:924–935.

42. Kuan TS, Tsou JY, Su FC. Hemiplegic gait of stroke patients: the effect of using a cane. Arch Phys Med Rehabil. 1999; 80:777–784.

43. Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2006; 22:51–56.

44. Siegel KL, Kepple TM, Stanhope SJ. Joint moment control of mechanical energy flow during normal gait. Gait Posture. 2004; 19:69–75.

45. Bobath K, Bobath B. The facilitation of normal postural reactions and movements in the treatment od cerebral palsy. Physiotherapy. 1964; 50:246–262.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download