Abstract
Purpose
We evaluated hemodynamic significance of stenosis on magnetic resonance angiography (MRA) using acetazolamide perfusion single photon emission computed tomography (SPECT).
Materials and Methods
Of 171 patients, stenosis in internal carotid artery (ICA) and middle cerebral artery (MCA) (ICA-MCA) on MRA and cerebrovascular reserve (CVR) of MCA territory on SPECT was measured using quantification and a 3-grade system. Stenosis and CVR grades were compared with each other, and their prognostic value for subsequent stroke was evaluated.
Results
Of 342 ICA-MCA, 151 (44%) presented stenosis on MRA; grade 1 in 69 (20%) and grade 2 in 82 (24%) cases. Decreased CVR was observed in 9% of grade 0 stenosis, 25% of grade 1, and 35% of grade 2. The average CVR of grade 0 was significantly different from grade 1 (p<0.001) and grade 2 stenosis (p=0.007). In quantitative analysis, average CVR index was -0.56±7.91 in grade 0, -1.81±6.66 in grade 1 and -1.18±5.88 in grade 2 stenosis. Agreement between stenosis and CVR grades was fair in patients with lateralizing and non-lateralizing symptoms (κ=0.230 and 0.346). Of the factors tested, both MRA and CVR were not significant prognostic factors (p=0.104 and 0.988, respectively), whereas hypertension and renal disease were significant factors (p<0.05, respectively).
Stenosis in the carotid or cerebral artery is a major cause of hemodynamic disturbance and subsequent stroke in the brain.123 In recent years, magnetic resonance angiography (MRA) has been increasingly used as a non-invasive imaging modality for evaluating carotid arteries and intracranial cerebral arteries.4 On MRA of ischemic stroke patients, abnormality is frequently observed in the internal carotid artery (ICA) and middle cerebral artery (MCA).5 Severity of intracranial stenosis evaluated on MRA is also applicable to guide revascularization or pharmacologic treatment.67 Additionally, long-term follow-up using MRA was reported to be effective in ischemic stroke patients with intracranial atherosclerosis.8 Although the application of MRA to cerebrovascular disease is still relatively limited due to various factors, including cost and socioeconomic factors,9 MRA is commonly used as the first diagnostic imaging method in patients suspected with cerebrovascular disease or even in patients with vague neurologic symptoms.
However, stenosis in the ICA or MCA that is not related to any neurologic symptom is often observed on MRA. In such cases, assessment of cerebral hemodynamic state can give additional and complementary information. Single photon emission computed tomography (SPECT) for brain perfusion has been widely used for assessing cerebrovascular reserve (CVR), which can be measured by rest and acetazolamide SPECT.10 Brain perfusion and CVR are more direct indices for hemodynamic state than vascular stenosis. CVR can be used for identifying patients who are at higher risk of ischemic stroke without surgical revascularization,11 and also for predicting prognosis in acute stage of stroke.12 As a prognostic factor inducing subsequent stroke, however, image findings of MRA and SPECT have not been fully understood.
The purpose of this study was to investigate the hemodynamic significance of the carotid or cerebral artery stenosis detected on MRA. For this purpose, vascular stenosis on MRA was compared with CVR on brain perfusion SPECT. Additionally, clinical significance of the image findings were evaluated in terms of prognosis for subsequent stroke, in comparison with other established clinical risk factors.
From the image database of our institution, patients who underwent both MRA and brain perfusion SPECT between 2009 and 2011 were retrospectively enrolled in this study. Among them, the following criteria were used for selection: 1) time interval between MRA and SPECT less than 60 days, 2) absence of previous cerebral infarction in the MCA territory, and 3) clinical follow-up after imaging study for more than 3 months.
Clinical information was obtained from thorough review of medical records, in terms of demographic information, neurologic symptoms, and events of stroke. When a patient had lateralizing neurologic symptoms such as unilateral weakness, paralysis, and paresthesia, symptoms were attributed to ischemia in one of the bilateral MCA territory. Non-lateralizing symptoms such as sudden explosive headache, syncope and collapse, dizziness and giddiness, or pathologic amnesia were attributed to both sides of hemispheres. A patient was regarded asymptomatic when there was no significant neurologic symptom at the time of MRA and SPECT, and during at least 1-month follow-up. The occurrence of stroke was determined based on follow-up medical records and brain imaging studies, and the onset date of new ischemic symptoms was regarded as the date of stroke occurrence.
The design of this study and exemption of the informed consent were approved by the Institutional Review Board of our institution.
Brain magnetic resonance image (MRI), including time-offlight (TOF)-MRA, was performed using a 3-T machine (Sigma, GE Medical Systems, Milwaukee, WI, USA) and an 8-channel head coil. The target MCA for high-resolution MRI was determined based on review of TOF-MRA images. The black-blood technique with preregional fat saturation pulses of 80-mm thickness was used to saturate incoming arterial flow. The highresolution MRI sequences included T1-weighted images (repetition time, 600 ms; echo time, 12 ms), T2-weighted images (repetition time, 2910 ms; echo time, 70 ms), proton density images (repetition time, 2500 ms; echo time, 30 ms), and T1-weighted images with gadolinium enhancement. All images were obtained with the following parameters: field of view, 120×120 mm; slice thickness, 2 mm; matrix size, 384×269; and number of excitations, 4.13
MRA images were reviewed by an experienced neuroradiologist, in terms of the ICA and MCA. The severity of a stenotic lesion of the ICA was measured according to the formula of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) method, in which % stenosis was calculated as [1-(minimum residual lumen diameter/distal internal carotid lumen diameter)]×100.141516 The % stenosis of MCA was defined as [1-(narrowest diameter/adjacent normal diameter)]×100, where the adjacent normal diameter was measured at a point distal to the stenosis, or at a point proximal to the stenosis in case of total occlusion. Because the MCA territory was the main target of analysis, stenosis in the ipsilateral ICA and/or MCA (ICA-MCA) was assessed using a 3-grade system: 0, no stenosis; 1, a single lesion of mild to moderate stenosis (<75% stenosis); 2, a single lesion of severe stenosis (≥75% stenosis) or multiple lesions. The cutoff of 75% was adopted from the NASCET, in which a stenosis over 70% was related to an increased risk of subsequent stroke. Aneurysms in the cerebral arteries or intracranial/extracranial carotid arteries were also evaluated on MRA.
Basal/acetazolamide brain perfusion SPECT was performed using one-day protocol. Basal SPECT images were acquired 5 minutes after intravenous injection of 555 MBq 99mTc-hexamethylproplyeneamine oxime (HMPAO), using a triple-headed gamma camera (Triad XLT 9, Trionix Research Laboratory, Twinsburg, OH, USA) equipped with low-energy ultrahigh-resolution fanbeam collimators. Forty step-and-shoot images were acquired for 20-25 seconds per step, with intervals of 3°. Ten minutes before the end of the basal SPECT, 20 mg/kg of acetazolamide was injected intravenously. Another dose of 99mTc-HMPAO (1.11 GBq) was injected at the end of basal SPECT, and second acquisition of SPECT was started 5 minutes later without position change. Acetazolamide SPECT images were acquired by decay-corrected subtraction of the basal images from the second SPECT images. All SPECT images were reconstructed on 128×128 matrix using a filtered backprojection method with a Butterworth filter.
Basal/acetazolamide SPECT images were assessed based on consensus of two experienced nuclear medicine specialists blinded to patient history, MRI findings, and final diagnoses. CVR in an MCA territory was assessed using a 3-grade system: 0, no decrease; 1, mild decrease (involving less than one-third of any lobe); and 2, severe decrease (involving more than onethird of any lobe).17
For quantitative analysis, SPM2 software (Wellcome Trust Centre for Neuroimaging, University College London, London, UK) implemented on MATLAB 7.6 (MathWorks, Natick, MA, USA) was used. As a preprocessing procedure, SPECT images were spatially normalized to a standard SPECT template (Montreal Neurological Institute, McGill University) using affine and nonlinear transformation. To define regional volume of interest (VOI), we used a statistical probabilistic anatomic map that provides probabilistic VOIs for various brain regions, including the cerebellum and bilateral MCA territories.18 The mean count of the each MCA territory was normalized to that of the cerebellum. Cerebrovascular reserve index (CVRI) in each MCA territory was defined as (Cacetazolamide-Cbasal)/(Cbasal)×100, where Cbasal and Cacetazolamide are the normalized counts on basal and acetazolamide SPECT, respectively.
Statistical analysis was performed using a commercial statistics package (MedCalc 9.5, MedCalc Software, Mariakerke, Belgium). Chi-square test was used for group comparisons between MRA and SPECT results. Kappa statistics were calculated for evaluating inter-rate agreement. To evaluate prognostic factors for subsequent stroke, logistic regression was performed and Kaplan-Meier survival curve was analyzed to evaluate probability of subsequent stroke. p values less than 0.05 was regarded statistically significant.
A total of 171 patients (108 men and 63 women, age 66±13 years) were included in the analysis, and 111 patients exhibited neurologic symptoms at the time of imaging studies. Patients had various risk factors for cerebral ischemia such as hypertension, hyperlipidemia, diabetes, smoking, ischemic heart disease, and previous episodes of transient ischemic attack (TIA). Some patients had underlying diseases related to cerebral artery diseases. The patient characteristics are summarized in Table 1.
In 171 patients, 342 cases of bilateral ICA-MCA and matched MCA territories were analyzed. On MRA, 191 (56%) cases exhibited no stenosis and 151 (44%) exhibited stenosis; grade 1 in 69 (20%) and grade 2 in 82 (24%) (Table 2). On perfusion SPECT, 278 (81%) cases exhibited normal CVR and 64 (19%) exhibited decreased CVR; grade 1 in 41 (12%) and grade 2 in 23 (7%). Decreased CVR was observed in 9% of grade 0 stenosis, 25% of grade 1, and 35% of grade 2 (Fig. 1). The average CVR grades were 0.3±0.6 in grade 1 stenosis and 0.5±0.7 in grade 2 stenosis, which were significantly higher than that of grade 0 stenosis (0.1±0.4; p<0.001 and p=0.007, respectively).
However, most of MCA territories with grade 1 and 2 stenosis on MRA presented normal CVR (75% and 65%, respectively). On the quantitative analysis, the average CVRI values were -0.56±7.91 in grade 0 stenosis, -1.81±6.66 in grade 1, and -1.18±5.88 in grade 2. The differences in quantitative values were not statistically significant. The inter-rate agreement between stenosis grade and CVR grade was poor (κ=0.192) in overall patients. However, the agreement was fair in patients with lateralizing symptoms (κ=0.230) or non-lateralizing symptoms (κ=0.346).
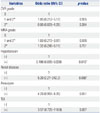
During follow-up of 18.7±9.6 months (median 20.0 months), 9 events of stroke occurred in 7 patients (Table 3). The incidence was 4.1% and the time interval between imaging studies and stroke was 5-27 months (Fig. 2). CVR grade and stenosis grade were not significantly different between patients with and without subsequent stroke (0.3±0.6 vs. 0.2±0.4, p=0.864 for CVR grade; 0.7±0.8 vs. 0.8±1.0, p=0.726 for stenosis grade). Of the clinical and imaging parameters tested, both stenosis and CVR grades were not significant prognostic factors, regardless of grade (Table 4). Hypertension and renal disease were significant prognostic factors for predicting subsequent stroke. Additionally, aneurysm and TIA were prognostic factors with borderline significance (Fig. 3).
In this study, we observed a significant correlation between ICA-MCA stenosis on MRA and CVR of MCA territory on perfusion SPECT. However, a considerable discrepancy between stenosis and CVR also existed; 75% of grade 1 stenosis and 65% of grade 2 stenosis were not matched with CVR impairment. And, significant prognostic factors were different according to symptomatic and asymptomatic groups. Severe stenosis on MRA (grade 2) and combination imaging factor of decreased CVRI and MRA grade 2 were significant predictors to cause subsequent stroke in lateralizing symptomatic patients.
MRA has been performed for evaluating vascular malformation,19 as well as major cerebrovascular occlusion,2021 whereas perfusion SPECT has been used for diagnosis and prognosis prediction in cerebrovascular diseases.22232425 Acetazolamide SPECT can provide information on CVR in addition to basal cerebral perfusion. There have been several reports on the use of CVR for evaluating clinical significance of cerebrovascular stenosis. A meta-analysis by Gupta, et al.26 showed that highgrade ICA stenosis or occlusion causing CVR impairment can be a risk factor for ischemic stroke. Stenosis on MRA also has a clinical significance, and Chen, et al.27 reported that degree of stenosis on MRA was inversely correlated with cerebrovascular reactivity in response to increased concentration of inspired CO2. Deteriorated autoregulation due to stenosis may cause hemodynamic impairment.
However, in the present study, only limited agreement existed between stenosis and CVR, although there was a significant difference in CVR according to stenosis grade. Additionally, most of the stenosis in ICA-MCA did not cause CVR impairment. It is most likely due to the difference in patient population, because we enrolled consecutive patients who met the inclusion criteria. Many patients in this study presented with no or only vague neurologic symptoms, and stenosis was incidentally detected in some of them. In such cases, stenosis may have been well tolerated and compensated by sufficient collateral flow. Intriguingly, in patients with any lateralizing and non-lateralizing neurologic symptoms, CVR impairment exhibited a fair agreement with stenosis (κ=0.230 and κ=0.346). Similarly, it has been reported that stenosis of carotid arteries is not significantly correlated with the hemispheric symptoms.28 Thus, it should be noted that stenosis in ICA-MCA may not be clinically significant in many asymptomatic patients.
In the present study, both the stenosis and CVR were found not to be significant prognostic factors for subsequent stroke, in contrast to previous reports.112930 The low event rate (9 events in 7 patients, 7/171=4.1%) in this study would have been one of the causes for the result. In addition, it could be attributed to the characteristics of our patient population. Because stroke can occur due to either thrombosis or embolism, stenosis in ICA or MCA is not the only cause for the stroke event. In a recent study, it was reported that approximately 47% of acute ischemic stroke had no prior occlusion within 1 month of the event and was classified as embolic occlusion.31 In the present study, 5 cases exhibited normal MRA, 6 cases exhibited normal CVR and 4 cases exhibited normal CVRI in 9 event cases, which suggests the probability of embolism as the cause for stroke. Therefore, it is speculated that clinical factors exhibited stronger prognostic power for the subsequent stroke in this study. Our results are in line with previous reports in which obesity, smoking,32 end-stage renal disease,33 and cerebral aneurysm34 are significant risk factors for stroke. However, hypertension exhibited an opposite result, which may result from our classification for hypertension. Because the current state of hypertension control was not considered in this study, mild or well-controlled hypertension was all included in the group of hypertension. It may have been a cause for selection bias.
The results of this study suggest that the carotid or cerebral artery stenosis in asymptomatic patients should be interpreted in terms of hemodynamic significance. Even in severe stenosis group of grade 2, more than half of the stenosis did not cause CVR impairment. Thus, evaluation of CVR using brain perfusion SPECT or other modality would be required for re-evaluation of stenotic lesions, particularly when MRA is used for screening of cerebrovascular disease in asymptomatic patients. However, further study is required to determine the diagnostic roles of brain perfusion SPECT and MRA in more homogeneous group of asymptomatic patients or patients with high risk factors for stroke.
There are several limitations in this study. First, ICA and MCA were analyzed together as a single group on MRA, whereas CVR was analyzed for MCA territory on perfusion SPECT. Although both ICA and MCA stenosis can lead to CVR impairment in the MCA territory, the influence of specific locations of stenosis was not considered in the analysis. Second, in some patients, the time interval between MRA and perfusion SPECT was somewhat long, up to 60 days. However, the time interval was less than 2 weeks in 74% of the patients. Third, this was a retrospective study and the patient group was relatively heterogeneous in terms of symptoms, underlying diseases, medications, and risk factors. Because we attempted to analyze the hemodynamic significance of stenosis on MRA in various conditions, we included all the consecutive patients.
In conclusion, a considerable proportion of ICA-MCA stenosis detected on MRA did not cause CVR impairment, although there was a fair correlation between them. Thus, it is recommended that hemodynamic state should be assessed for evaluation of stenotic lesions, particularly in asymptomatic patients.
Figures and Tables
 | Fig. 1Proportions of decreased CVR (grade 1 and 2) according to stenosis grade. Decreased CVR was more frequently presented in higher stenosis grade group. CVR, cerebrovascular reserve. |
 | Fig. 3Kaplan-Meier survival curve for subsequent stroke occurrence according to significant risk factors (A and B) or factors with borderline significances (C and D). TIA, transient ischemic attack. |
Table 1
Clinical Characteristics of 171 Patients

Table 2
CVR State of MCA Territories According to MRA Findings
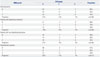
Table 3
Clinical Characteristics of Patients Who Presented Subsequent Stroke

HT, hypertension; DL, dyslipidemia; DM, diabetes mellitus; IHD, ischemic heart disease; SM, smoking; TIA, transient ischemic attack; LRF, liver or renal failure; MI, myocardial infarction; AF, atrial fibrillation; MCA, middle cerebral artery; R MCA, right MCA; L MCA, left MCA; CVRI, cerebrovascular reserve index; CVR, cerebrovascular reserve; MRA, magnetic resonance angiography.
Table 4
Prognostic Factors for Subsequent Stroke
ACKNOWLEDGEMENTS
This study has been presented as a poster in The Korea Society of Nuclear Medicine (Oct. 25th. 2012).
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2009-0093820).
References
1. Zhang Y, Wu S, Jia Z, Zhou Y, Liu X, Wang W, et al. The relationship of asymptomatic intracranial artery stenosis and Framingham stroke risk profile in a Northern Chinese industrial city. Neurol Res. 2012; 34:359–365.
2. Yang F, Liu L, Li M, Li M, Yin Q, Guo R, et al. Pattern of cerebrovascular atherosclerotic stenosis in older Chinese patients with stroke. J Clin Neurosci. 2013; 20:979–983.

3. Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000; 283:2122–2127.

4. Gao T, Yu W, Liu C. Mechanisms of ischemic stroke in patients with intracranial atherosclerosis: a high-resolution magnetic resonance imaging study. Exp Ther Med. 2014; 7:1415–1419.

5. Kumar G, Kalita J, Kumar B, Bansal V, Jain SK, Misra U. Magnetic resonance angiography findings in patients with ischemic stroke from North India. J Stroke Cerebrovasc Dis. 2010; 19:146–152.

6. Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005; 26:1012–1021.
7. Lutsep HL, Clark WM. Association of intracranial stenosis with cortical symptoms or signs. Neurology. 2000; 55:716–718.

8. Man BL, Fu YP, Chan YY, Lam W, Hui CF, Leung WH, et al. Use of magnetic resonance angiography to predict long-term outcomes of ischemic stroke patients with concurrent stenoses in Hong Kong. Cerebrovasc Dis. 2009; 28:112–118.

9. Lee K, Kim H, Heo JH, Bae HJ, Koh IS, Chang S. Application of magnetic resonance imaging and magnetic resonance angiography as diagnostic measures for the first attack of suspected cerebrovascular diseases in Korea. Yonsei Med J. 2011; 52:727–733.

10. Park SA, Park HI, Kim D, Yang CY, Zhang LQ. The prediction of gross motor outcome using cerebrovascular reserve measured by acetazolamide-challenged SPECT. NeuroRehabilitation. 2012; 30:359–367.

11. Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it. Stroke. 2001; 32:2110–2116.

12. Marchal G, Bouvard G, Iglesias S, Sebastien B, Benali K, Defer G, et al. Predictive value of (99m)Tc-HMPAO-SPECT for neurological Outcome/Recovery at the acute stage of stroke. Cerebrovasc Dis. 2000; 10:8–17.

13. Kim JM, Jung KH, Sohn CH, Moon J, Han MH, Roh JK. Middle cerebral artery plaque and prediction of the infarction pattern. Arch Neurol. 2012; 69:1470–1475.

14. Moore WS, Barnett HJ, Beebe HG, Bernstein EF, Brener BJ, Brott T, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation. 1995; 91:566–579.
15. Nederkoorn PJ, van der Graaf Y, Eikelboom BC, van der Lugt A, Bartels LW, Mali WP. Time-of-flight MR angiography of carotid artery stenosis: does a flow void represent severe stenosis. AJNR Am J Neuroradiol. 2002; 23:1779–1784.
16. U-King-Im JM, Trivedi RA, Cross JJ, Higgins NJ, Hollingworth W, Graves M, et al. Measuring carotid stenosis on contrast-enhanced magnetic resonance angiography: diagnostic performance and reproducibility of 3 different methods. Stroke. 2004; 35:2083–2088.

17. So Y, Lee HY, Kim SK, Lee JS, Wang KC, Cho BK, et al. Prediction of the clinical outcome of pediatric moyamoya disease with postoperative basal/acetazolamide stress brain perfusion SPECT after revascularization surgery. Stroke. 2005; 36:1485–1489.

18. Lee HY, Paeng JC, Lee DS, Lee JS, Oh CW, Cho MJ, et al. Efficacy assessment of cerebral arterial bypass surgery using statistical parametric mapping and probabilistic brain atlas on basal/acetazolamide brain perfusion SPECT. J Nucl Med. 2004; 45:202–206.
19. Raoult H, Bannier E, Maurel P, Neyton C, Ferré JC, Schmitt P, et al. Hemodynamic quantification in brain arteriovenous malformations with time-resolved spin-labeled magnetic resonance angiography. Stroke. 2014; 45:2461–2464.

20. Kimura K, Iguchi Y, Shibazaki K, Aoki J, Uemura J. Early recanalization rate of major occluded brain arteries after intravenous tissue plasminogen activator therapy using serial magnetic resonance angiography studies. Eur Neurol. 2009; 62:287–292.

21. Schellinger PD, Richter G, Kohrmann M, Dorfler A. Noninvasive angiography (magnetic resonance and computed tomography) in the diagnosis of ischemic cerebrovascular disease. Techniques and clinical applications. Cerebrovasc Dis. 2007; 24:Suppl 1. 16–23.

22. Deus-Silva L, Bonilha L, Damasceno BP, Costa AL, Yasuda CL, Costa FF, et al. Brain Perfusion Impairment in Neurologically Asymptomatic Adult Patients with Sickle-Cell Disease Shown by Voxel-Based Analysis of SPECT Images. Front Neurol. 2013; 4:207.

23. Oikawa K, Ogasawara K, Saito H, Yoshida K, Saura H, Sato Y, et al. Combined measurement of cerebral and cerebellar blood flow on preoperative brain perfusion SPECT imaging predicts development of new cerebral ischemic events after endarterectomy for symptomatic unilateral cervical carotid stenosis. Clin Nucl Med. 2013; 38:957–961.

24. Volkan-Salanci B, Lay Ergün E, Genc Sel Ç, Yalnizoğlu D, Turanli G. The role of brain perfusion SPECT in Moyamoya disease. Rev Esp Med Nucl Imagen Mol. 2012; 31:216–218.

25. Saura H, Ogasawara K, Suzuki T, Kuroda H, Yamashita T, Kobayashi M, et al. Effect of combination therapy with the angiotensin receptor blocker losartan plus hydrochlorothiazide on brain perfusion in patients with both hypertension and cerebral hemodynamic impairment due to symptomatic chronic major cerebral artery steno-occlusive disease: a SPECT study. Cerebrovasc Dis. 2012; 33:354–361.

26. Gupta A, Chazen JL, Hartman M, Delgado D, Anumula N, Shao H, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke. 2012; 43:2884–2891.

27. Chen J, Liu J, Xu WH, Xu R, Hou B, Cui LY, et al. Impaired dynamic cerebral autoregulation and cerebrovascular reactivity in middle cerebral artery stenosis. PLoS One. 2014; 9:e88232.

28. Kanber B, Hartshorne TC, Horsfield MA, Naylor AR, Robinson TG, Ramnarine KV. Wall motion in the stenotic carotid artery: association with greyscale plaque characteristics, the degree of stenosis and cerebrovascular symptoms. Cardiovasc Ultrasound. 2013; 11:37.

29. Liu M, Zhou L. Cerebrovascular reserve may be a more accurate predictor of stroke than degree of ICA or MCA stenosis. Med Sci Monit. 2014; 20:2082–2087.

30. Kuroda S, Shiga T, Ishikawa T, Houkin K, Narita T, Katoh C, et al. Reduced blood flow and preserved vasoreactivity characterize oxygen hypometabolism due to incomplete infarction in occlusive carotid artery diseases. J Nucl Med. 2004; 45:943–949.
31. Lee KJ, Jung KH, Byun JI, Kim JM, Roh JK. Infarct pattern and clinical outcome in acute ischemic stroke following middle cerebral artery occlusion. Cerebrovasc Dis. 2014; 38:31–38.

32. Itrat A, Ahmed B, Khan M, Muhammad M, Thaver D, Khowaja Z, et al. Risk factor profiles of South Asians with cerebrovascular disease. Int J Stroke. 2011; 6:346–348.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download