Abstract
Purpose
To investigate the effects of resveratrol on the expression of hypoxia-inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF) in human adult retinal pigment epithelial (ARPE-19) cells, and on experimental choroidal neovascularization (CNV) in mice.
Materials and Methods
ARPE-19 cells were treated with different concentrations of resveratrol and then incubated under hypoxic conditions with subsequent evaluation of cell viability, expression of HIF-1α, and expression of VEGF. The effects of resveratrol on the synthesis and degradation of hypoxia-induced HIF-1α were evaluated using inhibitors of the PI3K/Akt/mTOR and the ubiquitin proteasome pathways. In animal studies, CNV lesions were induced in C57BL/6 mice by laser photocoagulation. After 7 days of oral administration of resveratrol or vehicle, which began one day after CNV induction, image analysis was used to measure CNV areas on choroidal flat mounts stained with isolectin IB4.
Results
In ARPE-19 cells, resveratrol significantly inhibited HIF-1α and VEGF in a dose-dependent manner, by blocking the PI3K/Akt/mTOR signaling pathway and by promoting proteasomal HIF-1α degradation. In mice experiments, orally administered resveratrol significantly inhibited CNV growth in a dose-dependent manner.
Pathological neovascularization plays a key role in the pathophysiology of various diseases including cancers, heart diseases, and ocular diseases. In the eye, pathological neovascularization is associated with leading causes of blindness in all age groups; retinopathy of prematurity in children, diabetic retinopathy in adults, and age-related macular degeneration (AMD) in the elderly.1 Blocking neovascularization using vascular endothelial growth factor (VEGF) inhibitors has been successfully employed in the clinic to treat these sight-threatening diseases,2345 but anti-VEGF therapies are still ineffective in significant number of patients, especially in AMD patients.
A master regulator of the hypoxic response is the hypoxiainducible factor (HIF)-1, a heterodimeric transcription factor composed of an α-subunit whose proteasomal degradation is tightly regulated by oxygen tension and constitutively expressed β-subunit.6 Under hypoxic conditions, the HIF-1 complex binds hypoxia response elements at promoters and enhancers of target genes, and induces expression of a wide array of pro-angiogenic molecules including VEGF, platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF).7 Because the HIF pathway is a master regulator of angiogenesis, and modulates multiple pro-angiogenic pathways, it represents an attractive target for anti-angiogenic therapy. Furthermore, HIF-1 has been found to be expressed in human choroidal neovascularization (CNV) membrane, which is a pathognomonic feature of neovascular type AMD, and retinal pigment epithelial (RPE) cells.89
Resveratrol (trans-3,4,5'-trihydroxystibene), a naturally occurring polyphenolic compound found in grapes and red wine, has received a great deal of attention due to its beneficial roles involving anti-oxidant, anti-inflammatory, anti-aging, and anti-carcinogenic activities.1011 Studies have also shown that resveratrol potently suppresses VEGF in various cancer cells, possibly via inhibition of HIF-1α.121314 In the present study, we demonstrated that resveratrol can inhibit expression and secretion of VEGF by inhibition of HIF-1α in human RPE cells under hypoxic conditions. The inhibitory effects of orally administered resveratrol on laser-induced CNV in a mouse model were also investigated.
Resveratrol was purchased from Sigma-Aldrich (St. Louis, MO, USA). LY294002, PD98059, and SB203528 were purchased from Calbiochem (San Diego, CA, USA). The protein synthesis inhibitor, cycloheximide, and the proteasome inhibitor, MG132 (Z-leu-leu-CHO), were purchased from Calbiochem (Darmstadt, Germany). Primary antibody to HIF-1α was purchased from BD Transduction Laboratories (San Diego, CA, USA). Antibodies to phosphorylated Akt1/2/3 (Ser437) and total Akt 1/2 (N-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Primary antibodies to phosphorylated mTOR (Ser2448) and total mTOR were purchased from Cell Signaling Technology (Beverly, MA, USA). Rapamycin and everilomus were purchased from Sigma-Aldrich.
Human retinal pigment epithelial cells (ARPE-19) were purchased from ATCC (Manassas, VA, USA). Cells were cultured in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 µg/mL).
Cells were cultured to -80% confluency, and then transferred to hypoxic conditions, using a modulator incubator chamber (Billups-Rothenberg, Del Mar, CA, USA), which was flushed for 10 minutes with a gas mixture containing 95% N2/5% CO2, resulting in a final concentration of 3% O2. The incubator chambers were sealed and placed in a traditional incubator at 37℃.
ARPE-19 cells, 70-80% confluent in complete medium, were pretreated for 1 hour with different concentrations of resveratrol (10 µmol/L, 50 µmol/L, and 100 µmol/L), followed by incubation under hypoxic conditions for 4 or 16 hours. To determine the effects of resveratrol on the half-life or degradation of hypoxia-induced HIF-1α protein, ARPE-19 cells were pretreated for 1 hour with 10 µg/mL cycloheximide or 20 µmol/L of MG132 and cultured in the presence or absence of 100 µmol/L resveratrol for 6 hours under hypoxic conditions.
Total RNA was isolated from ARPE-19 cells using Ribospin (GeneAll, Inc., Seoul, Korea). Reverse transcription-polymerase chain reaction (RT-PCR) analysis of VEGF and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels were determined using the M-MLV kit (Invitrogen) with primers specific for HIF-1α (forward primer, 5'-TCACCACAGGACAGTACAG GATGC-3'and reverse primer, 5'-CCAGCAAAGTTAAAGCAT CAGGTTCC-3'); for human VEGF (forward primer, 5'-ACG AAGTGGTGAAGTTCATGGATG-3' and reverse primer, 5'-TTC TGTATCAGTCTTTCCTGGTGAG-3'); and for human GAPDH (forward primer, 5'-GCCAAAGGGTCATCATCTC-3' and reverse primer, 5'-GTAGAGGCAGGGATGATGTT-3'). All the primers were synthesized by Genolution (Genolution, Seoul, Korea).
Cells were washed once with cold phosphate-buffered saline (PBS), solubilized with lysis buffer [40 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.5), 120 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, and 1 tablet of EDTA free protease inhibitor cocktail (Roche, Indianapolis, IN, USA)]. The lysed samples were centrifuged at 20817×g (14000 rpm) for 1 hour at 4℃ to obtain the supernatant. Total protein in the supernatant was quantified using the bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) using bovine serum albumin as a standard. The proteins were blotted onto polyvinylidene difluoride (PVDF) membranes (Millipore Co., Bedford, MA, USA) after separation by 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE). The membranes were blocked for 1 hour with 10% non-fat dry milk (Noble Bio, Hwaseong, Korea) in Tris-buffered saline containing 0.1% Tween-20 (TBS-T), and incubated overnight with primary antibody at 4℃. The membranes were then washed 4 times with TBS-T for 10 minutes each at room temperature, and incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour. The blots were developed using the ECL chemiluminescence reagent kit (Santa Cruz Biotechnology and Millipore).
ARPE-19 cells were plated onto 48-well plates at 2.5×105 cells per well. After pretreatment with different concentrations of resveratrol for 1 hour, cells were placed under hypoxic conditions for 16 hours. Viable cells were determined using the MTT assay and the In Vitro Toxicology Assay Kit, Neutral Red based (Sigma-Aldrich), according to the manufacturer's instructions.
To determine VEGF secretion by ARPE-19 cells under hypoxic conditions, culture medium was assayed for VEGF using the Quantikine human VEGF immunoassay (Koma Biotech, Inc., Seoul, Korea), according to the manufacturer's instructions.
ARPE-19 cells were seeded into 100-mm cell culture dishes, transfected with either siRNA for the human mTOR gene, or with randomly scrambled siRNA (both synthesized by Genolution), using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. The human mTOR siRNA contained specific target sequences (sense, 5'-CCCUGCCUU UGUCAUGCCUUU-3' and antisense, 5'-AGGCAUGACAAAG GCAGGGUU-3'), and the scrambled siRNA contained the sequences (sense, 5'-ACGUGACACGUUCGGAGAAUU-3' and antisense 5'-UUCUCCGAACGUGUCACGUUU-3'). The cells were transfected with an additional 20 nM/100 mm dish of mTOR siRNA or scrambled siRNA for 4 hours, followed by a change of medium and incubation for 48 hours, and then incubated under hypoxic conditions for 16 hours. The effects of siRNA treatment were determined using western blot analysis, luciferase assays, and RT-PCR.
All mice were handled in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Protocols were approved by the Institutional Animal Care and Use Committee of Yonsei University, Seoul, Korea. CNV was induced by laser photocoagulation as previously described.15 Briefly, nine-weekold male C57BL/6 mice were anesthetized with intraperitoneal tiletamine-zolazepam (30 mg/kg, Zoletil; Virbac, Carros, France) and xylazine (10 mg/kg; Rompun, Bayer Korea Ltd., Seoul, Korea). Mice pupils were dilated with 0.5% tropicamide and 0.5% phenylephrine (Mydrin-P; Santen Pharmaceutical Co., Osaka, Japan). Argon green laser photocoagulation (100 µm spot size, 180 mW power, and 0.1 second duration, Ultima 2000 SE, Lumenis, Santa Clara, CA, USA) was performed with a slit lamp delivery system. Three to four laser burns were made in each eye at the 3, 6, 9, and 12 o'clock positions, approximately 2 disc diameters from the optic nerve. Only laser burns that produced a cavitation bubble, which indicates the rupture of Bruch's membrane, were included in the study.
Resveratrol is a white solid powder, soluble in 10% ethanol/90% H2O at room temperature.
The resveratrol solution was thawed immediately prior to oral administration. The mice were randomized into three groups: 1) a control group with vehicle only (10% ethanol/90% H2O), 2) a low dose group with 1 mg/kg/day resveratrol, and 3) a high dose group with 10 mg/kg/day resveratrol. Each drug was administered one day after laser photocoagulation and daily given once thereafter.
After 7 days of oral administration of drugs, 24 eyes (8 eyes from 4 mice per group) were subjected to choroidal flat mounts. The eyes were enucleated and immediately fixed in 4% paraformaldehyde in PBS for 1 hour. Under a stereoscopic microscope, the anterior segments were removed, and the neurosensory retinas were detached and separated from the optic nerve head. After 4 radial incisions, the eyecups were washed with cold PBlec solution [0.1 mM CaCl2, 0.1 mM MgCl2, 0.1 mM MnCl2, and 1% Triton X-100 in PBS (pH 6.8)] by overnight incubation at 4℃. The eyecups were incubated overnight with a 1:1000 dilution of a 10 mg/mL solution of 4',6-diamidino-2-phenylindole (DAPI), and a 1:100 dilution of a 1 mg/mL solution of isolectin IB4 conjugated with Alexa Fluor 488 (Invitrogen-Molecular Probes, Eugene, OR, USA) in a cold chamber at 4℃, followed by washing with cold PBS and then flat-mounted with the RPE side facing up.1617 The samples were covered and sealed for confocal microscopic analysis (LSM 700META, Carl Zeiss, Thornwood, NY, USA) at 488 nm (FITC) and at 405 nm (DAPI).
All statistical parameters were calculated using GraphPad Prism 5.0 software. The results were analyzed by one-way analysis of variance. The size of the CNV lesions was evaluated by ANOVA, followed by the Dunnett's post hoc test. Differences with a p-value less than 0.05 were considered statistically significant.
Resveratrol treatment via oral feeding for 1 week did not induce any significant side effects, including weight loss, infection, and death. The recipient mice maintained normal appetite and activity. However, resveratrol treatment resulted in a significant reduction in the area of laser-induced CNV on day 7. An image analysis of CNV on choroidal flat mounts showed that the median (mean±SD) CNV areas of the vehicle group, low dose group (1 mg/kg), and high dose group (10 mg/kg) were 21607 (29730±12940) µm2, 13432 (17184±10835) µm2, and 9005 (12186±6320) µm2, respectively. There was 37.8% (42.2%, for the mean value) decrease in the median CNV size in the low dose group, and 58.3% (59.0%, for the mean value) decrease in the high dose group, compared to the vehicle group. The differences were statistically significant for both low dose (p<0.001) and high dose groups (p<0.001) (Fig. 1).
To determine whether resveratrol could inhibit VEGF expression in human ARPE-19 cells under hypoxic conditions, we determined VEGF mRNA expression by using RT-PCR, and the level of VEGF secretion using by ELISA. The results showed that treatment of ARPE-19 cells with resveratrol resulted in a dosedependent decrease of VEGF expression at both mRNA and protein levels (Fig. 2A and B). The cell viability assays indicated that the decrease of VEGF expression by resveratrol was not due to cell death.
Pretreatment of ARPE-19 cells with resveratrol abrogated the hypoxia-induced HIF-1α protein accumulation in a dose-dependent manner (Fig. 2C). HIF-1α expression and its downstream target gene expressions have been found to be regulated by major signal transduction pathways, including PI3K/Akt and ERK/MAPK.1819 To determine whether these pathways were involved, ARPE-19 cells were pretreated with specific inhibitors of PI3K (50 µM LY294002), ERK1/2 (50 µM PD98059), and p38 MAPK (20 µM SB203528) for 1 hour, followed by incubation under hypoxic conditions. Pretreatment of ARPE-19 cells with LY294002 suppressed HIF-1α protein accumulation and VEGF mRNA expression, suggesting the involvement of PI3K/Akt activation in HIF-1α expression and its downstream VEGF gene expression in ARPE-19 cells (data not shown). To determine whether resveratrol inhibited hypoxia-mediated Akt phosphorylation and its downstream mammalian target of mTOR phosphorylation, ARPE-19 cells were pretreated with varying concentrations of resveratrol for 1 hour, followed by hypoxic incubation for 6 hours, and then the levels of phospho-Akt and phospho-mTOR were analyzed using western blots. The results showed that pretreatment with resveratrol resulted in a dose-dependent decrease of both phospho-Akt and phospho-mTOR levels (Fig. 2D).
The mTOR molecule is one of the downstream targets of PI3K/Akt, which regulates signaling proteins which are important for protein synthesis, such as p70S6 kinase.20 ARPE-19 cells were pretreated with mTOR inhibitors, rapamycin and everolimus, followed by incubation under hypoxic conditions. As shown in Fig. 3A, these treatments resulted in a decrease in hypoxia-induced HIF-1α accumulation, along with abrogation of phospho-p70S6 kinase levels, the downstream target of mTOR (Fig. 3A). As a complementary strategy, we attempted to silence the mTOR gene using siRNA. Transfection of ARPE-19 cells with an mTOR siRNA effectively reduced hypoxia-induced HIF-1α protein accumulation, mTOR levels, phospho-mTOR protein levels, and phospho-p70S6 kinase levels (Fig. 3B). Silencing of mTOR using siRNA also resulted in inhibition of hypoxia-induced VEGF secretion (Fig. 3C).
HIF-1α protein levels are regulated by a balance between its synthesis and degradation. Hypoxia induces HIF-1α protein accumulation mainly by promoting its stability, instead of increasing its synthesis.21 To test whether resveratrol can inhibit hypoxia-induced HIF-1α protein accumulation by promoting its degradation, ARPE-19 cells were exposed to hypoxia for 4 hours, followed by treatment with cycloheximide to block ongoing protein synthesis in the presence or absence of resveratrol for various time periods. Hypoxia-induced HIF-1α protein accumulation gradually decreased to an undetectable level at 120 minutes following exposure to cycloheximide, whereas in the absence of resveratrol, HIF-1α protein levels remained relatively stable (Fig. 4A and B). To further test whether the degradation of hypoxia-induced HIF-1α protein promoted by resveratrol was mediated by ubiquitination and 26S proteasomemediated degradation, ARPE-19 cells were treated with 26S proteasome inhibitor MG132 (20 µM) for 1 hour in the absence or presence of resveratrol. Inhibition of HIF-1α protein expression by resveratrol was significantly blocked in the presence of MG132, suggesting that the degradation of hypoxia-induced HIF-1α protein mediated by resveratrol was dependent on the proteasome degradation pathway in ARPE-19 cells (Fig. 4C).
Resveratrol is a naturally occurring compound that has been of great interest because of its beneficial effects, including anti-aging, anti-obesity, anti-inflammatory, and anti-carcinogenic activities.101222 The effect of resveratrol on angiogenesis is complex; it has been shown to be pro-angiogenic in the ischemic myocardium, but anti-angiogenic in cancer cells.121323 In the eye, resveratrol has been shown to inhibit proliferation of hypoxic choroidal vascular endothelial cells.24 We found that resveratrol inhibited VEGF secretion via an HIF-1 dependent pathway in human RPE cells, and inhibited CNV growth in laser-induced CNV mice.
In ARPE-19 cells, HIF-1α protein expression under hypoxia was found to be dependent on the PI3K/Akt/mTOR pathway. HIF-1α protein level is also tightly regulated by oxygen tension via ubiquitination and 26S proteasomal degradation system.25 Under hypoxic conditions, degradation of HIF-1α protein via ubiquitination is inhibited, leading to stabilization and accumulation of HIF-1α protein. We found that resveratrol shortened the half-life of hypoxia-induced HIF-1α protein (Fig. 4A and B). This effect was significantly reduced in the presence of MG132, a 26S proteasome inhibitor (Fig. 4C). These results are consistent with previous studies of cancer cells,1213 suggesting that resveratrol inhibits HIF-1α protein levels by regulating both protein translation and protein degradation. This dual effect may explain why there was still a mild inhibition of HIF-1α accumulation by resveratrol in the presence of MG132 (Fig. 4C). Although HIF-1α degradation by resveratrol was blocked, inhibition of HIF-1α translation by resveratrol can still be possible via PI3K/Akt/mTOR pathway.
The laser injury-induced mouse CNV model has been widely used for studying neovascular AMD.26 In this animal model, oral administration of resveratrol markedly inhibited CNV progression in a dose-dependent manner. Analyses of choroidal flat mounts demonstrated a mean 42.2% reduction of CNV areas in the low dose group, and a mean 59.0% reduction in the high dose group (Fig. 1). Consistent with these results, a previous studies found a significant reduction in laser-induced mouse CNV using systemic administration of resveratrol or grape power via implanted osmotic pumps or oral gavage.2728 However, in the above studies, administration of resveratrol or grape power began 5 to 7 days before laser-induced injury and continued after CNV induction. In the present study, however, oral administration of resveratrol began one day after laser induced injury and continued for 7 days thereafter, nevertheless, significant CNV suppression was achieved, further providing evidence for its therapeutic potential in the management of CNV. Prolonged pre-treatment with resveratrol could have resulted in improved anti-angiogenic response. The anti-angiogenic effect of resveratrol has been found in other pathological angiogenesis models of ocular disease, as well. Resveratrol inhibited retinal neovascularization in very low-density lipoprotein receptor knock-out mice and diabetes-induced early vascular lesions in mouse retina.2930
In summary, our study has provided both in vitro and in vivo evidence that resveratrol exerts anti-angiogenic effects on CNV through its potent inhibition of HIF-1α via PI3K/Akt/mTOR pathway. This finding suggests a therapeutic value of resveratrol in AMD patients, possibly as an adjunct oral therapy to current intravitreal anti-VEGF treatments. The anti-angiogenic properties of resveratrol might also be exploited to treat various ocular disorders and other diseases that involve abnormal angiogenesis.
Figures and Tables
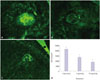 | Fig. 1Resveratrol inhibited choroidal neovascularization (CNV) growth in animal model. Effects of orally-administered resveratrol on lesion size in laser-induced CNV mice at Bruch membrane rupture sites. Representative photographs of choroidal flat mounts 7 days after laser photocoagulation are shown: (A) control group, (B) low dose group (1 mg/kg/day), and (C) high dose group (10 mg/kg/day). (D) Image analysis shows reduced CNV size from the resveratrol-treated mice. *p<0.001 and †p<0.0001 vs. the vehicle-treated control. |
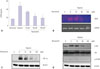 | Fig. 2Resveratrol inhibited hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF) via the PI3K/Akt pathway. ARPE-19 cells in complete medium were pretreated with 10 µmol/L, 50 µmol/L, and 100 µmol/L resveratrol for 1 hour, and then exposed to normoxia or hypoxia for 16 hours. Resveratrol significantly reduced VEGF protein and mRNA levels as determined by ELISA (A) and RT-PCR (B) in a dose-dependent manner. (C) Pretreatment with resveratrol in varying concentration abrogated the hypoxia-induced HIF-1α protein accumulation in a dose-dependent manner. (D) Pretreatment with resveratrol resulted in decrease of phospho-Akt (p-Akt) and phospho-mTOR (p-mTOR) levels in a dose-dependent manner. *p<0.0001 vs. the normoxia group, †p<0.001 vs. the hypoxia group. ELISA, Enzyme-Linked Immunosorbent Assay. |
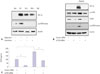 | Fig. 3(A) Inhibition of the mTOR pathway using rapamycin and everolimus prevented HIF-1α induction under hypoxic conditions in ARPE-19 cells as determined by immunoblotting of HIF-1α and p-p70S6 kinase. (B) Transfection of ARPE-19 cells with human mTOR siRNA resulted in HIF-1α, phosphomTOR, mTOR, and phospho-p70S6 kinase as determined by western blot. (C) Silencing of mTOR using siRNA resulted in inhibition of hypoxia-induced vascular endothelial growth factor (VEGF) in ARPE-19 cells. *p<0.0001 vs. the control group. HIF, hypoxia-inducible factor; p-mTOR, phospho-mTOR. |
 | Fig. 4ARPE-19 cells were exposed to hypoxia for 4 hours, followed by treatment with cycloheximide (CHX) in the absence (A) or presence (B) of 100 µmol/L of resveratrol (RSV), for different time periods, and then analyzed by western blotting for HIF-1α protein. Hypoxia-induced HIF-1α protein accumulation gradually decreased to an undetectable level at 120 minutes in the presence of resveratrol (B). (C) Inhibition of hypoxia-induced HIF-1α protein accumulation by resveratrol was significantly blocked in the presence of MG132, a 26S proteasome inhibitor. HIF, hypoxia-inducible factor. |
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Jeong Hun Kim and Dr. Dong Hyun Jo [Fight against Angiogenesis-Related Blindness (FARB) laboratory], Seoul National University College of Medicine, Seoul, Korea for kind support in establishing a laser CNV mouse model. This study was supported by a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A110272), and a faculty research grant of Yonsei University College of Medicine for 2011 (6-2011-0068).
References
1. Hyman LG, Lilienfeld AM, Ferris FL 3rd, Fine SL. Senile macular degeneration: a case-control study. Am J Epidemiol. 1983; 118:213–227.

2. Mintz-Hittner HA, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011; 364:603–615.

3. Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.

4. Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2007; 91:1318–1322.

5. Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010; 33:2399–2405.

6. Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol. 2000; 59:47–53.

7. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985). 2000; 88:1474–1480.
8. Inoue Y, Yanagi Y, Matsuura K, Takahashi H, Tamaki Y, Araie M. Expression of hypoxia-inducible factor 1alpha and 2alpha in choroidal neovascular membranes associated with age-related macular degeneration. Br J Ophthalmol. 2007; 91:1720–1721.

9. Sheridan CM, Pate S, Hiscott P, Wong D, Pattwell DM, Kent D. Expression of hypoxia-inducible factor-1alpha and -2alpha in human choroidal neovascular membranes. Graefes Arch Clin Exp Ophthalmol. 2009; 247:1361–1367.
10. Juhasz B, Varga B, Gesztelyi R, Kemeny-Beke A, Zsuga J, Tosaki A. Resveratrol: a multifunctional cytoprotective molecule. Curr Pharm Biotechnol. 2010; 11:810–818.

11. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997; 275:218–220.

12. Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J, Le AD. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol Cancer Ther. 2005; 4:1465–1474.

13. Cao Z, Fang J, Xia C, Shi X, Jiang BH. trans-3,4,5'-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004; 10:5253–5263.

14. Park SY, Jeong KJ, Lee J, Yoon DS, Choi WS, Kim YK, et al. Hypoxia enhances LPA-induced HIF-1alpha and VEGF expression: their inhibition by resveratrol. Cancer Lett. 2007; 258:63–69.

15. Chung EJ, Yoo S, Lim HJ, Byeon SH, Lee JH, Koh HJ. Inhibition of choroidal neovascularisation in mice by systemic administration of the multikinase inhibitor, sorafenib. Br J Ophthalmol. 2009; 93:958–963.

16. Kang S, Roh YJ, Kim IB. Antiangiogenic effects of tivozanib, an oral VEGF receptor tyrosine kinase inhibitor, on experimental choroidal neovascularization in mice. Exp Eye Res. 2013; 112:125–133.

17. Kang S, Park KC, Yang KJ, Choi HS, Kim SH, Roh YJ. Effect of cediranib, an inhibitor of vascular endothelial growth factor receptor tyrosine kinase, in a mouse model of choroidal neovascularization. Clin Experiment Ophthalmol. 2013; 41:63–72.

18. Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002; 64:993–998.

19. Minet E, Michel G, Mottet D, Raes M, Michiels C. Transduction pathways involved in Hypoxia-Inducible Factor-1 phosphorylation and activation. Free Radic Biol Med. 2001; 31:847–855.

20. Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, et al. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol. 2009; 8:25–33.
22. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006; 444:337–342.

23. Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005; 39:813–822.

24. Balaiya S, Murthy RK, Chalam KV. Resveratrol inhibits proliferation of hypoxic choroidal vascular endothelial cells. Mol Vis. 2013; 19:2385–2392.
25. Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998; 95:7987–7992.

26. Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010; 51:2813–2826.

27. Khan AA, Dace DS, Ryazanov AG, Kelly J, Apte RS. Resveratrol regulates pathologic angiogenesis by a eukaryotic elongation factor-2 kinase-regulated pathway. Am J Pathol. 2010; 177:481–492.

28. Kanavi MR, Darjatmoko S, Wang S, Azari AA, Farnoodian M, Kenealey JD, et al. The sustained delivery of resveratrol or a defined grape powder inhibits new blood vessel formation in a mouse model of choroidal neovascularization. Molecules. 2014; 19:17578–17603.

29. Kim YH, Kim YS, Roh GS, Choi WS, Cho GJ. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012; 90:e31–e37.

30. Hua J, Guerin KI, Chen J, Michán S, Stahl A, Krah NM, et al. Resveratrol inhibits pathologic retinal neovascularization in Vldlr(-/-) mice. Invest Ophthalmol Vis Sci. 2011; 52:2809–2816.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download