Abstract
The risk of radiotherapy-related secondary cancers in children with constitutional retinoblastoma 1 (RB1) mutations has led to reduced use of external beam radiotherapy (EBRT) for RB. Presently, tumor reduction with chemotherapy with or without focal surgery (chemosurgery) is most commonly undertaken; EBRT is avoided as much as possible and is considered only as the last treatment option prior to enucleation. Nevertheless, approximately 80% of patients are diagnosed at a locally advanced stage, and only 20-25% of early stage RB patients can be cured with a chemosurgery strategy. As a whole, chemotherapy fails in more than two-thirds of eyes with advanced stage disease, requiring EBRT or enucleation. Radiotherapy is still considered necessary for patients with large tumor(s) who are not candidates for chemosurgery but who have visual potential. When radiation therapy is indicated, the lowest possible radiation dose combined with systemic or local chemotherapy and focal surgery may yield the best clinical outcomes in terms of local control and treatment-related toxicity. Proton beam therapy is one EBRT method that can be used for treatment of RB and reduces the radiation dose delivered to the adjacent orbital bone while maintaining an adequate dose to the tumor. To maximize the therapeutic success of treatment of advanced RB, the possibility of integrating radiotherapy at early stages of treatment may need to be discussed by a multidisciplinary team, rather than considering EBRT as only a last treatment option.
Radiation oncologists face difficulties with using radiotherapy to treat children with retinoblastoma (RB). External beam radiotherapy (EBRT), which was long used with great success to completely control these tumors, was found to also save globes and vision. However, radiation was found to increase the life-long risk of secondary cancers in children with constitutive RB1 mutations. Radiation was also found to affect the growth of the soft tissue and bone around the eye. At present, radiotherapy is usually performed when all other treatments have failed or when tumors are large and cannot be controlled by focal surgery.
The ultimate goals of RB treatment are to save life and vision, as well as the eye itself. Every effort must be made to preserve vision, regardless of whether RB is unilateral or bilateral. Focal surgical treatment, such as cryotherapy, thermotherapy, or laser therapy, can control the tumor, save vision, and preserve cosmesis when tumors are at an early stage. The selection of a treatment modality for RB patients depends on several factors, including age at diagnosis, the risk of secondary malignancy after treatment, the site of the tumor within the globe, the clinical stage of the tumor, and the visual potential of the involved eye after treatment.
Since only 20% of RB patients have a family history of this disease,1 most patients with RB cannot be diagnosed via a screening examination. Therefore, most RBs are detected only when there are symptoms and signs, and the majority of patients are diagnosed at advanced intraocular stages. About 80% of patients seen at ophthalmology clinics present with large tumors or with subretinal and/or vitreous seeding.2 Radiotherapy is still an important treatment option in these patients when there is a possibility of saving the eyeball and vision. The risk of metastasis increases, while the likelihood of saving useful vision decreases, when the tumor invades the optic nerve, choroid, or orbit. In such cases, timely enucleation reduces the risk of metastatic spread, the side-effects of chemotherapy and radiotherapy, and the need for repeat examinations under anesthesia.23 In general, secondary glaucoma, pars plana seeding, and anterior chamber invasion are also best managed by enucleation.1
There are great disparities in the survival rates of children with RB among different regions of the world. Patient mortality rates range from 3-5% in Europe, Canada, and the US and 40-70% in certain parts of Asia and Africa.4 These differences are largely due to delayed diagnosis and the lower priority of health care for rare diseases such as RB in less developed countries.5 The Reese-Ellsworth (RE) classification (Table 1) and the International Classification of RB (ICRB) (Table 2) are the most commonly used methods of classifying RB limited to the orbit, and are frequently utilized in developed countries where extraocular disease is relatively rare. In contrast, American Joint Committee on Cancer TNM staging takes into account systemic disease and includes the statuses of both extraocular and intraocular involvement (Table 3). The RE classification system was created in 1963 to predict rates of tumor control and globe preservation following photon radiation therapy using lateral beams. Peripheral tumors were given a higher stage due to technical difficulties in preserving vision using lateral beam EBRT. The RE classification divides tumors into five groups and 10 subgroups, based on the size, location, and number of tumors (Table 1). Due to the more frequent use of chemotherapy in the 1990s, the ICRB system was designed to better predict responses to chemotherapy. Although the ICRB system is based on tumor size and location, it also classifies tumors by the presence or absence of subretinal and vitreous seeds, as well as by the extent of retinal involvement, indicated as the percentage of the total retinal area (Table 2). Tumors staged in the high ICRB group are regarded as involving a high risk of chemotherapy failure and thus potentially requiring EBRT or enucleation. Therefore, the ICRB system can predict globe, but not systemic, prognosis.
Ninety-five percent of RB gene mutations can be detected by clinically available methods. Bilaterality is considered a surrogate marker of heritability, and age less than 12 months at the time of diagnosis is likely to be associated with heritability. About 15% of patients with unilateral RB, whose parents have no ophthalmologic abnormality, exhibit genetic mutations. Family history is absent in 80% of children with RB, with the mutation occurring de novo. Hence, genetic testing is recommended for all patients with RB, as it enables prenatal diagnosis of RB 1 mutations for the children of RB survivors. If an RB 1 gene mutation is detected, the fetus can be screened for genetic mutations during gestation. Fetuses with RB gene mutations are recommended to undergo post-natal ophthalmologic examinations every three months, enabling early detection. Second and subsequent children should also be tested if an older sibling was diagnosed with RB.16 Early treatment of small tumors found by regular screening after birth enables both complete tumor removal and preservation of vision. Genetic testing of pediatric patients with unilateral RB is also recommended, as awareness of mutation status can assist in predicting the risk of secondary malignancies. If a mutation test is negative, the patient's parents can be advised that radiotherapy is not a hazardous treatment option and that the risk of having another affected child is negligible.1 Moreover, information about the absence of a gene mutation can obviate the need for the patients' siblings and relatives to undergo repeated invasive surveillance procedures under anesthesia.
Tumors with RB gene mutations appear to be more sensitive to radiation. Previously, control of RB tended to be more successful in patients with bilateral rather than unilateral RB; this was attributed to the fact that patients with bilateral disease are more likely to have RB gene mutations. Of 110 eyes with vitreous seeds treated with high dose chemotherapy and periocular carboplatin, 33 failed treatment and required EBRT, whereas 77 were salvaged. A multivariate analysis of factors prognostic for tumor regression showed that bilateral RB and the absence of subretinal fluid were predictive of salvage, suggesting that RB-mutated cells are more sensitive to chemotherapy and/or radiotherapy.7 In support of this clinical observation, RB pathway inactivation in breast cancer was shown to be associated with an improved response to neoadjuvant chemotherapy.8 A genomic study of drug sensitivity in cancer showed that cells with RB gene alterations were more sensitive to mitotic inhibitors such as paclitaxel.9 In vitro assessments of RB cell lines showed that they were extremely sensitive to ionizing radiation.10 Animal RB tumors also showed significantly greater radiosensitivity than control tumors at doses of 17.5 proton Gray (cobalt gray equivalent, CGE) or above.11 Moreover, successful treatment outcomes were observed when low dose radiotherapy, ranging from 24-36 Gy, was administered as the primary treatment121314 or as salvage treatment after chemotherapy,1516 suggesting that there is a subset of RBs that are exceptionally sensitive to radiation.
Focal therapies, including laser therapy, thermotherapy, cryotherapy, and plaque radiotherapy, are used to treat patients initially diagnosed with group A RB, according to the ICRB. Plaque radiotherapy using radioisotopes, such as iodine 125 and ruthenium 106, can be considered for the treatment of groups B or C RBs when the tumor diameter is <16 mm, tumor thickness is 4-9 mm, and vitreous seeding is limited.17 Plaque radiotherapy is an excellent treatment for small isolated tumors located far from the optic nerve or macula, as well as for tumors recurring focally after chemotherapy or EBRT.
Chemotherapy used to be the main modality for tumors with extraocular extension invading the choroid, optic nerve, and orbit, as well as for tumors with systemic metastasis. Since the 1990s, however, chemotherapy has been widely used as a primary treatment for locally advanced RB to reduce tumor size prior to focal therapies. Since even long-term systemic chemotherapy is ineffective at controlling intraocular RB when used on its own, good initial responses to chemotherapy in most patients must be consolidated with laser photocoagulation, cryotherapy, and thermotherapy.4 This strategy, called chemoreduction or chemosurgery, has become the standard approach for intraocular tumors of all stages. Chemosurgery on locally advanced RB is performed to avoid EBRT, control the tumor, and save vision in that eye. However, over 80% of tumors are either too large at presentation or have subretinal and/or vitreous seeding, preventing the application of this strategy.2 Fewer than 25% of RBs are cured by chemotherapy with or without focal techniques,2 with most cured patients classified as having ICRB groups A and B tumors. Moreover, this treatment fails in 40% of group C eyes and the majority of groups D and E eyes, necessitating enucleation or radiation. Overall, approximately 47% of patients with advanced disease still require EBRT.1819
The effect of chemoreduction on 14 patients with RE group V RB found that chemotherapy coupled with EBRT of 40-44 Gy over 20-22 fractions salvaged 67% of eyes with vitreous seeding.2021 However, the functional outcomes of the salvaged eyes were not good.
Systemic chemotherapy, however, is often associated with toxicities. Permanent hearing loss has been reported in 5-33% of children, which is especially detrimental for those who have problems with vision. The RB database of the National Institutes of Health of the USA and Memorial Sloan-Kettering Cancer Center reported that 15 patients developed a secondary malignancy, particularly acute myelogenous leukemia, which was fatal in 10 of these 15 patients.222 Chemotherapy administered via the ophthalmic artery [intra-arterial chemotherapy (IAC)] has become the most widely used type of local chemotherapy. IAC was developed to reduce the toxicity of systemic chemotherapy and to avoid EBRT. IAC has been shown to be effective in locally controlling 80-100% of patients with groups D and E RB when used in combination with systemic chemotherapy.20 Systemic chemotherapy is required as the primary therapy in higher risk RB, which includes bilateral groups D and/or E. It could provide prevention of metastatic disease as well as control of intraocular tumors. IAC could be a new option for unilateral RB and possesses little risk of systemic complication and mostly associates with local ocular toxicities such as ptosis, choroidal vascular attenuation, and optic neuropathy.20 Periocular injection of carboplatin via the subconjuctival or sub-Tenon's space is being attempted, although its long-term toxicity has not yet been determined. Also, intravitreal chemotherapy using melphalan or methotrexate has been used for recurrent RB with vitreous seeding; however, its long-term complications have not been assessed.
The use of EBRT to treat RB has decreased dramatically over the past four decades, more than for other types of pediatric cancer. According to the National Cancer Institute's Surveillance, Epidemiology, and End Results database of the nine original tumor registries (SEER-9), the use of EBRT for RB has declined from 30% of treatments in the period from 1973 to 1976 to 2% in the period from 2005 to 2008.23 An evaluation of 595 patients who were treated between 1973 and 2009 showed that enucleation rates remained stable from 1990 to 2000,24 suggesting that the eye preservation rates in the chemoreduction era were not improved, compared with the EBRT era. According to that report, EBRT was delivered as part of initial treatment to 21.5% of all RB patients, including 51.6% of patients with bilateral disease and 10.7% of those with unilateral disease.24 Enucleation of the more severely affected eye and irradiation of the other eye is still a common practice in treating patients with bilateral RB. EBRT rather than local therapy is also used to treat patients with multifocal RB and those with tumors close to the macular or optic nerve with preserved vision. EBRT is also used to treat large tumors and those with vitreous seeding that do not respond to systemic chemotherapy. Tumors too large or difficult to treat with radiotherapy alone may be treated with combinations of radiotherapy and focal surgical procedures to optimize cure rates and reduce the risks of treatment-related complications that may result from moderate to high dose radiotherapy. EBRT was most frequently used in the 1980s, when about 30% of patients with RB were treated with this modality.24
Conventional EBRT in the megavoltage era showed local control rates of 41-56%, with eye survival rates of 60-100%.1325262728 Local control rates were reported to be 78.5% for RE groups I-II eyes and 20% for RE groups III-V eyes.28 Failure may occur in 40-60% of patients, with salvage with other focal modalities, resulting in long-term eye survival rates of around 80%. Eye survival was also found to be correlated with clinical stage, ranging from 80-90% for RE groups I-III to 60% for RE groups IV-V.26 The correlation between tumor stage and local control rate is consistent among studies; however, the relationship between tumor size and local control rate remains unclear. One study reported that failure rates at the primary site differed for tumors <15 mm and >15 mm in diameter (50% vs. 21%),28 whereas other studies did not observe clear differences in the dose-response relationships for varying tumor sizes.2527 Complications of EBRT include dryness of the eye, cataract, and orbital hypoplasia. During the megavoltage EBRT era, cataract developed in about 20-30% of eyes252829 about 2-3 years after radiotherapy. The incidence of post-radiotherapy cataract is higher in patients treated with orthovoltage X-rays.30 Glaucoma, neovascularization, and hemorrhage sometimes require enucleation after EBRT. Today, in the chemoreduction era, where radiotherapy is used as a last resort after all other treatments have failed, the complication rate after EBRT is likely to be much higher than that for previous eras,2 since patients referred for radiotherapy have already undergone multi-agent systemic and regional chemotherapy and multiple sessions of focal surgery. Chemotherapy and EBRT may synergize in producing long-term complications, such as retinal and optic disc ischemia, similar to findings in patients with central nervous system tumors.31
At present, the clinical endpoints for RB treatment include the rates of avoidance of EBRT, as well as rates of local control and eye preservation. The results of chemoreduction are summarized in Table 4. Although chemoreduction achieved considerable tumor control in early stage RB, treatment outcomes for patients with RE group VB and ICRB group E RB remain poor. A study of 101 eyes in 101 patients with unilateral RB (21 group C, 40 group D, 40 group E by ICRB) reported that intensified chemotherapy with periocular carboplatin resulted in eye salvage in 20 (95%) group C, 34 (85%) group D, and 23 (57.5%) group E eyes. They also found that 96% of salvaged eyes achieved visual acuity of 20/200 or better, with 33 patients requiring EBRT.7 An earlier study of EBRT alone found that the long-term globe salvage rate using doses of 42-45 Gy was 53%, with local control in 50% of patients with ICB group E and RE group VB RB.25 Salvage EBRT after chemotherapy and focal surgical therapies showed relatively good salvage rates, especially for patients with bilateral RB. For example, among 90 patients with bilateral RB who received primary chemotherapy and focal treatments, 36 eyes of 22 patients failed and required salvage EBRT. Of the 36 eyes, 24 eyes (66.7%) were controlled by EBRT of 40-44 Gy over 20-22 fractions and required no further treatment. Overall, 30 out of 36 eyes (83.3%)34 were preserved at 40 months. The likelihood of salvage was better in eyes with early stage RB, although salvage was observed in 28.6-62.5% of RE groups IV-V patients. Visual acuity was preserved in 19 eyes, although 31.6% read 6/60 or worse.34 Another study reported the treatment outcomes in 30 eyes of 15 patients with bilateral RB who had group D (RE group V) in at least one eye. Of the 18 group D eyes in patients treated with primary chemotherapy alone, two (11%) showed complete tumor control, seven (39%) underwent enucleation, and nine underwent successful salvage treatment. EBRT was a successful salvage treatment in five of these 9 patients, resulting in an event-free survival rate of 34% two years after treatment.35
The traditional therapeutic dose of EBRT is 40-50 Gy; however, successful tumor control has been reported with doses less than 36 Gy.1213 Patients treated with EBRT after cytoreduction with chemotherapy and repeated focal surgical therapies may be at greater risk for eye complications, while cytoreduction modalities may place patients at greater risks of vascular complications and drug toxicity.20 A lower dose of radiation may be considered when radiotherapy is used as a consolidation treatment followed by other treatment modalities. The rates of enucleation and therapeutic radiotherapy were reported to be significantly lower in patients treated with chemotherapy plus low-dose prophylactic planned EBRT than chemotherapy alone.16 In that study, patients who previously underwent enucleation of the contralateral eye and those with group E RB with no clinically visible recurrent tumors were offered EBRT 2600 cGy over 13 days, starting two months after chemotherapy. In contrast, patients with a normal contralateral eye and those with groups A-D RB were treated with chemoreduction with or without therapeutic EBRT of 4000 cGy over 20 days. Among the patients with group E RB, those managed with chemotherapy and prophylactic low-dose EBRT had a significantly lower recurrence rate, a lower likelihood of enucleation, and less of a need for high-dose therapeutic radiotherapy than patients managed with chemotherapy alone. The globe salvage rates of eyes managed with chemotherapy alone, chemotherapy plus therapeutic EBRT, and chemotherapy plus lower-dose prophylactic RT were 25%, 50%, and 83%, respectively. In another study, 18 patients (24 eyes) with group D RB were treated with chemoreduction, local treatment including plaque radiotherapy, sub-Tenon carboplatin injection, and 2400-3600 cGy intensity modulated radiotherapy (IMRT). All patients showed persistent or recurrent disease after treatment. At a mean follow-up of 63 months, 19 eyes (79%) were salvaged, four were enucleated due to tumor recurrence at 9-31 months following radiotherapy, and one underwent enucleation for a painful eye and optic nerve atrophy 53 months after radiotherapy. The overall one- and five-year eye survival rates were 82% and 68%, respectively, with salvage radiotherapy with low dose IMRT, accounting for the preservation of an additional 35% of eyes. However, 12 eyes (50%) developed cataracts, which required extraction; four (17%) developed radiation retinopathy; and three (13%) developed retinal detachment requiring a scleral buckling procedure.15 Of the 36 patients who received salvage radiotherapy with 4000-4400 cGy/20-22 fractions after chemoreduction and focal therapies, 12 experienced tumor recurrence and six required enucleation. Twenty-four patients (66.7%) showed local control, with 30 eyes (83.3%) preserved after 40 months. Complications included keratoconjunctivitis sicca and cataract in four patients with no retinopathy.34 Taken together, these reports indicate that salvage EBRT with low dose radiotherapy may result in less orbital hypoplasia and better functionally preserved eyes. However, the local control rate was lower when compared with the same dose of EBRT as that of consolidation treatment.16 The comparison of chemoreduction, chemoreduction combined with EBRT, and chemoreduction combined with prophylactic lower dose RT is summarized in Table 5.
Modern precision radiotherapy techniques, such as IMRT and stereotactic radiotherapy using a hypofractionated dose schedule, aim to achieve conformal dose distribution to the eye tumor. However, IMRT results in a high integral dose by delivering low doses to surrounding tissues, which may increase the risk of secondary tumors. The volume of the bony orbit receiving >5 Gy was found to be 69% for IMRT, 25% for three-dimensional (3D) conformal electrons, and 10% for proton radiotherapy.38 A dosimetric study using 10 modern radiotherapy techniques showed that the volume of the ipsilateral bony orbit receiving at least 20 Gy (V20 GY) was much lower for arc-based IMRT than for 3D-conformal radiotherapy (56% vs. 90%).39
By reducing the radiation dose to the bone surrounding the eye, proton beam therapy (PBT) is expected to reduce the incidence of secondary sarcoma. A retrospective analysis of patients with RB treated with PBT at Massachusetts General Hospital or photon RT at Boston Children's Hospital showed that the former significantly reduced the rate of secondary malignancy [0/55 (0%) vs. 4/31 (13%)]40 (Fig. 1). However, since the median follow-up period was shorter for PBT than for photon RT (6.9 years vs. 13.1 years) and the number of RB patients treated with PBT was relatively small,40 this finding requires confirmation in larger patient cohorts.
Treating the entire retina due to concerns about new retinal lesions after EBRT was conventional practice.41 However, the rates of new lesions in the uninvolved retina were similar in patients who received focal and whole retinal treatment. Therefore, avoiding irradiation of the uninvolved retina may reduce the rates of eye complications.28 Whole retina treatment may be required for group D eyes as well as salvage therapy in eyes with vitreous or subretinal seeding unresponsive to chemotherapy. However, the anterior chamber can be excluded from the radiation field when the tumors are located in the posterior part of the globe, because small lesions occurring after PBT can be controlled with cryotherapy or laser therapy.40
Several proton beam delivery techniques are used to treat RB,338 the most frequent being the use of a single lateral beam or anterior oblique beam.38 The latter has the advantage of sparing the orbital bone while fully covering the retina. It may be difficult to save the posterior surface of the lens when the tumor is located anterior to the equator and when the retina at the level of the ora serrata requires irradiation. At the National Cancer Center, Korea, a silicon suction contact lens with a radio-opaque ring marker is placed on the cornea, and the eyeball is rotated to the nasal or temporal side, depending on the location of the tumor, so that the bone surrounding the orbit is not in the path of the proton beam (Fig. 2). Since the radiation dose to the surrounding bone and soft tissue is lower, PBT may reduce secondary cancer rates. This technique is generally associated with improved cosmetic results and good eye function (Fig. 3). Our institute uses a single scattering mode rather than a double scattering mode to treat the retina, as the former results in a reduced lateral penumbra and a smaller distal range than the latter. Although the Paul Sherrer Institute uses scanned beams to treat the entire orbit in patients with advanced RB,3 it may be difficult to treat a small volume and at a shallow depth with pencil beam scanning (PBS) when only the retina requires irradiation. The status of eye fixation can be viewed in real-time on the computer monitor in the treatment control room so that any deviation from the initial set-up can be immediately corrected (Fig. 4). The video image is transferred from a small closed-circuit camera attached to the periphery of the aperture attached to the snout. Examples of tumor regression after PBT are shown in Figs. 5 and 6.
Expert consensus recommends that radiotherapy is indicated when other means of saving the eye, such as chemotherapy and focal therapy, have failed.6 When radiotherapy is used, modern high-precision radiotherapy is recommended to minimize the dose to the orbital bones,6 and PBT could be an excellent treatment option. PBT can be used in combination with chemotherapy as a local treatment modality, consolidating the effect of chemoreduction, or as salvage treatment after other therapeutic modalities. Depending on the location of the tumor within the eye, proton beam dosimetry can minimize the dose to the orbital bone. Careful selection of patients may contribute to high cure rates with good vision and good cosmesis without the need for long-term chemotherapy and may reduce chemotherapy- and radiotherapy-associated complications. Patient selection should be based on careful examination to assess whether PBT can save the vision and eyes of patients with ICRB groups C-E RBs. Currently, there is no active multinational protocol for PBT of RB. The primary goals of PBT should be to save the patient's vision and eyeball, to prevent recurrence, and to increase overall survival rates. Secondary goals should include minimizing rates of secondary malignancy, avoiding eye complications, and improving cosmesis.
Although the gold standard in pediatric cancer care is to treat the patient within the context of a clinical trial, few clinical trials have been performed for RB due to the rarity of the disease and the varying clinical factors among individuals with RB. Hence, there is no class A evidence from randomized clinical trials to guide treatment. Current guidelines are available from the Canadian RB Society and the International Society of Paediatric Oncology for Developing Countries (SIOP-PODC), depending on socioeconomic setting.642 Short-course chemotherapy is currently being tested in an international, multicenter clinical trial to reduce the risk for short-term and long-term toxic effects. Current ongoing clinical trials are summarized in Table 6.
In an effort to avoid radiotherapy-related toxicity, including secondary malignancy, chemotherapy, which was formerly used only for RBs with extraocular extension or systemic metastasis, is now regarded as a primary treatment modality, even in patients with locally advanced intraocular RB, to reduce tumor size prior to focal therapies. However, over 80% of tumors are too large or too advanced at presentation for this strategy. Thus, EBRT remains the primary treatment option to preserve the eye and vision in these patients. The use of EBRT in RB patients previously treated with multiple rounds of systemic and local chemotherapy, with or without focal surgery, may yield poorer treatment outcomes than its previous de novo use, as evaluated by cure and eye complication rates. With recent advances in RT techniques, such as IMRT and PBT, radiation could be delivered more safely with a reduced dose to adjacent normal organs, resulting in a dramatic reduction of late complications. Meticulous planning by a multidisciplinary team of EBRT, beginning at the initial stage of treatment, can optimize therapeutic outcomes in patients with RB.
Figures and Tables
 | Fig. 1Cumulative rates of (A) "in-field" or "radiation-induced" secondary malignancies and (B) all secondary malignancies in patients treated with proton beam therapy and photon radiotherapy. |
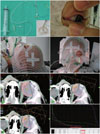 | Fig. 2Practices at National Cancer Center, Korea. (A) Under anesthesia, a small suction cup is placed on the cornea and the eyeball is rotated so that the proton beam can maximally avoid the orbital bone while covering the retinal target. (B) Dose distribution in proton beam therapy-initial field (left upper), boost field (right upper), summation of both fields (left lower) and corresponding dose volume histogram for the entire plan (right lower). |
 | Fig. 3Cosmetic outcomes in a patient treated with the radiotherapy plan described in Fig. 2. The patient's right eye was enucleated and the left eye was treated with PBT. Left to right: photos taken prior to PBT, 3 months after completion of PBT, and 6 years after PBT. PBT, proton beam therapy. |
 | Fig. 4Treatment monitoring system used at National Cancer Center, Korea. The position of the eye is monitored in the control room through a CC camera attached to the aperture during treatment. CC, closed-circuit. |
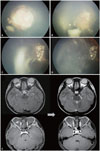 | Fig. 5Treatment outcome of unilateral RB after PBT. Fundoscopic examination of a 5-year-old boy diagnosed with RB in the right eye. (A) Pre-PBT: endophytic mass obscuring the posterior pole with two satellite masses. (B) During PBT: following the delivery of 2340 cGy, the main mass at the posterior pole has partially regressed, whereas the smaller seeding masses have slightly increased in size. (C) Two weeks after PBT: the main mass has regressed while the seeding masses have remained stable. (D) Three months after PBT: masses have overall remained stable. (E) MRI findings before (left) and after (right) PBT: a lobulated contoured intraocular mass of the right orbit, present before PBT, absent after PBT. RB, retinoblastoma; PBT, proton beam therapy. |
 | Fig. 6Treatment outcome of PBT in a patient with bilateral RB. The patient was diagnosed with bilateral RB and received chemotherapy and transpupillary thermotherapy (TTT) for 1 year. After treatment, the masses regressed and have remained stable for 1.5 years. However, new masses developed in the right eye. PBT was delivered to the mass refractory to TTT. (A) Pre-PBT, showing the re-growth of a solid mass (arrow) at the temporal margin of a previous mass that had regressed. (B, C, and D) Views 1 (B), 2.5 (C), and 8 (D) months after PBT, showing that the main mass had regressed and remained stable for 8 months. Five months after PBT, a hemorrhage developed at the site of the previously regressed mass treated with TTT, and persisted until 8 months after PBT. There has been no evidence of disease to date, but glaucoma developed as a result of hemorrhage. RB, retinoblastoma; PBT, proton beam therapy. |
Table 1
Reese-Ellsworth Classification of Intraocular Retinoblastoma

Table 2
International Classification for Retinoblastoma

Table 3
TNM Classification of Retinoblastoma

Table 4
Treatment Outcomes of Chemoreduction

| Authors | Patients | Chemotherapy | Focal treatment | Outcome* |
|---|---|---|---|---|
| Shields, et al.18 | 158 eyes of 103 patients (364 tumors) |
6 cycles (vincristine, etoposide, and carboplatin) |
Cryotherapy, thermotherapy, or plaque radiotherapy | Treatment failure rate at 5 yrs |
| RE groups I-IV: | ||||
| EBRT required in 10% | ||||
| Enucleation required in 15% | ||||
| RE group V: | ||||
| EBRT required in 47% | ||||
| Enucleation required in 53% | ||||
| Shield, et al.32 | 249 eyes of 163 patients |
6 cycles (vincristine, etoposide, and carboplatin) |
Thermotherapy or cryotherapy | Treatment success rate |
| ICRB group A: 100% | ||||
| ICRB group B: 93% | ||||
| ICRB group C: 90% | ||||
| ICRB group D: 47% | ||||
| Künkele, et al.33 | 56 eyes of 40 patients |
6 cycles (vincristine, etoposide, carboplatin, and cyclophosphamide) |
Thermotherapy, laser coagulation, cryotherapy, or brachytherapy | Treatment failure rates |
| ICRB group A: 25% | ||||
| ICRB group B: 15% | ||||
| ICRB group C: 33.3% | ||||
| ICRB group D: 83.3% |
Table 5
Comparison of Chemoreduction, Chemoreduction Plus Radiotherapy (RT) and Chemoreduction Plus Lower Dose Prophylactic RT in Advanced Retinoblastoma

| Treatment modality | Chemoreduction alone | Chemoreduction+RT | Chemoreduction+lower dose prophylactic RT |
|---|---|---|---|
| Pros | Avoid or delay enucleation or RT | Higher tumor control than chemoreduction alone or lower dose RT | Less recurrence than chemoreduction alone |
| Lower risk of RT related toxicity is expected than therapeutic RT | |||
| Cons | 30-50% eventually required RT for globe salvage18,28 | Late complication of radiation such as orbital bone hypoplasia or secondary malignancy | Exact risk of lower dose of RT is not known |
| Prospective study may needed | |||
| Globe salvage rate | Group D: 11-47%15,35 | RE groups IV-V: 28.6-62.5% at median F/U of 40 months34 | Group D: 82% at 1 yr, 68% at 5 yrs15 |
| Group E: 53% at 2 yrs, 48% at 5 yrs16 | Group E: 91% at 2 yrs, 80% at 5 yrs16 |
Table 6
Currently Active Protocols for Retinoblastoma (Modified from clinicaltrials.gov, as of Apr 2014)

References
1. Halperin EC, Constine LS, Tarbell NJ, Kun LE. Pediatric Radiation Oncology. Philadelphia: Wolters Kluwer Health;2012.
3. Munier FL, Verwey J, Pica A, Balmer A, Zografos L, Abouzeid H, et al. New developments in external beam radiotherapy for retinoblastoma: from lens to normal tissue-sparing techniques. Clin Experiment Ophthalmol. 2008; 36:78–89.

4. Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. Lancet. 2012; 379:1436–1446.

5. Dimaras H, Dimba EA, Gallie BL. Challenging the global retinoblastoma survival disparity through a collaborative research effort. Br J Ophthalmol. 2010; 94:1415–1416.

6. Canadian Retinoblastoma Society. National Retinoblastoma Strategy Canadian Guidelines for Care: Stratégie thérapeutique du rétinoblastome guide clinique canadien. Can J Ophthalmol. 2009; 44:Suppl 2. S1–S88.
7. Manjandavida FP, Honavar SG, Reddy VA, Khanna R. Management and outcome of retinoblastoma with vitreous seeds. Ophthalmology. 2014; 121:517–524.

8. Witkiewicz AK, Ertel A, McFalls J, Valsecchi ME, Schwartz G, Knudsen ES. RB-pathway disruption is associated with improved response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2012; 18:5110–5122.

9. Zhao J, Zhang Z, Liao Y, Du W. Mutation of the retinoblastoma tumor suppressor gene sensitizes cancers to mitotic inhibitor induced cell death. Am J Cancer Res. 2014; 4:42–52.
10. Zhang M, Stevens G, Madigan MC. In vitro effects of radiation on human retinoblastoma cells. Int J Cancer. 2001; 96:Suppl. 7–14.

11. Svitra PP, Budenz D, Albert DM, Koehler AM, Gragoudas E. Proton beam irradiation for treatment of experimental human retinoblastoma. Eur J Ophthalmol. 1991; 1:57–62.

12. Cassady JR, Sagerman RH, Tretter P, Ellsworth RM. Radiation therapy in retinoblastoma. An analysis of 230 cases. Radiology. 1969; 93:405–409.
13. Fontanesi J, Pratt CB, Hustu HO, Coffey D, Kun LE, Meyer D. Use of irradiation for therapy of retinoblastoma in children more than 1 year old: the St. Jude Children's Research Hospital experience and review of literature. Med Pediatr Oncol. 1995; 24:321–326.

14. Fontanesi J, Pratt C, Meyer D, Elverbig J, Parham D, Kaste S. Asynchronous bilateral retinoblastoma: the St. Jude Children's Research Hospital experience. Ophthalmic Genet. 1995; 16:109–112.

15. Berry JL, Jubran R, Kim JW, Wong K, Bababeygy SR, Almarzouki H, et al. Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr Blood Cancer. 2013; 60:688–693.

16. Shields CL, Ramasubramanian A, Thangappan A, Hartzell K, Leahey A, Meadows AT, et al. Chemoreduction for group E retinoblastoma: comparison of chemoreduction alone versus chemoreduction plus low-dose external radiotherapy in 76 eyes. Ophthalmology. 2009; 116:544–551.

17. Ramasubramanian A, Shields CL. Retinoblastoma. 1st ed. New Delhi: Jaypee Brothers Medical Publishers Ltd.;2012.
18. Shields CL, Honavar SG, Meadows AT, Shields JA, Demirci H, Singh A, et al. Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol. 2002; 133:657–664.

19. Shields CL, Mashayekhi A, Cater J, Shelil A, Meadows AT, Shields JA. Chemoreduction for retinoblastoma. Analysis of tumor control and risks for recurrence in 457 tumors. Am J Ophthalmol. 2004; 138:329–337.

20. Shields CL, Fulco EM, Arias JD, Alarcon C, Pellegrini M, Rishi P, et al. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye (Lond). 2013; 27:253–264.

21. Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol. 1996; 114:1339–1343.

22. Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology. 2007; 114:1378–1383.

23. Jairam V, Roberts KB, Yu JB. Historical trends in the use of radiation therapy for pediatric cancers: 1973-2008. Int J Radiat Oncol Biol Phys. 2013; 85:e151–e155.

24. Shinohara ET, DeWees T, Perkins SM. Subsequent malignancies and their effect on survival in patients with retinoblastoma. Pediatr Blood Cancer. 2014; 61:116–119.

25. Abramson DH, Beaverson KL, Chang ST, Dunkel IJ, McCormick B. Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol. 2004; 122:1316–1323.

26. Blach LE, McCormick B, Abramson DH. External beam radiation therapy and retinoblastoma: long-term results in the comparison of two techniques. Int J Radiat Oncol Biol Phys. 1996; 35:45–51.

27. Foote RL, Garretson BR, Schomberg PJ, Buskirk SJ, Robertson DM, Earle JD. External beam irradiation for retinoblastoma: patterns of failure and dose-response analysis. Int J Radiat Oncol Biol Phys. 1989; 16:823–830.

28. Hernandez JC, Brady LW, Shields JA, Shields CL, DePotter P, Karlsson UL, et al. External beam radiation for retinoblastoma: results, patterns of failure, and a proposal for treatment guidelines. Int J Radiat Oncol Biol Phys. 1996; 35:125–132.

29. Desjardins L, Chefchaouni MC, Lumbroso L, Levy C, Asselain B, Bours D, et al. Functional results after treatment of retinoblastoma. J AAPOS. 2002; 6:108–111.

30. Messmer EP, Fritze H, Mohr C, Heinrich T, Sauerwein W, Havers W, et al. Long-term treatment effects in patients with bilateral retinoblastoma: ocular and mid-facial findings. Graefes Arch Clin Exp Ophthalmol. 1991; 229:309–314.

31. Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet. 2009; 374:1639–1651.

32. Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006; 113:2276–2280.

33. Künkele A, Jurklies C, Wieland R, Lohmann D, Bornfeld N, Eggert A, et al. Chemoreduction improves eye retention in patients with retinoblastoma: a report from the German Retinoblastoma Reference Centre. Br J Ophthalmol. 2013; 97:1277–1283.

34. Chan MP, Hungerford JL, Kingston JE, Plowman PN. Salvage external beam radiotherapy after failed primary chemotherapy for bilateral retinoblastoma: rate of eye and vision preservation. Br J Ophthalmol. 2009; 93:891–894.

35. Cohen VM, Kingston J, Hungerford JL. The success of primary chemotherapy for group D heritable retinoblastoma. Br J Ophthalmol. 2009; 93:887–890.

36. Gündüz K, Shields CL, Shields JA, Meadows AT, Gross N, Cater J, et al. The outcome of chemoreduction treatment in patients with Reese-Ellsworth group V retinoblastoma. Arch Ophthalmol. 1998; 116:1613–1617.

37. Gündüz K, Günalp I, YalçindagXMLLink_XYZ N, Unal E, Taçyildiz N, Erden E, et al. Causes of chemoreduction failure in retinoblastoma and analysis of associated factors leading to eventual treatment with external beam radiotherapy and enucleation. Ophthalmology. 2004; 111:1917–1924.

38. Krengli M, Hug EB, Adams JA, Smith AR, Tarbell NJ, Munzenrider JE. Proton radiation therapy for retinoblastoma: comparison of various intraocular tumor locations and beam arrangements. Int J Radiat Oncol Biol Phys. 2005; 61:583–593.

39. Eldebawy E, Parker W, Abdel Rahman W, Freeman CR. Dosimetric study of current treatment options for radiotherapy in retinoblastoma. Int J Radiat Oncol Biol Phys. 2012; 82:e501–e505.

40. Sethi RV, Shih HA, Yeap BY, Mouw KW, Petersen R, Kim DY, et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. 2014; 120:126–133.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download