Abstract
Invasive aspergillosis (IA), generally considered an opportunistic infection in immunocompromised hosts, is associated with high morbidity and mortality. IA commonly occurs in the respiratory tract with isolated reports of aspergillosis infection in the nasal sinuses, central nervous system, skin, liver, and urinary tract. Extra-pulmonary aspergillosis is usually observed in disseminated disease. To date, there are a few studies regarding primary and disseminated gastrointestinal (GI) aspergillosis in immunocompromised hosts. Only a few cases of primary GI aspergillosis in non-immunocompromised hosts have been reported; of these, almost all of them involved the upper GI tract. We describe a very rare case of IA involving the lower GI tract in the patient without classical risk factors that presented as multiple colon perforations and was successfully treated by surgery and antifungal treatment. We also review related literature and discuss the characteristics and risk factors of IA in the immunocompetent hosts without classical risk factors. This case that shows IA should be considered in critically ill patients, and that primary lower GI aspergillosis may also occur in the immunocompetent hosts without classical risk factors.
Invasive aspergillosis (IA) usually occurs in immunocompromised hosts.1 Classical risk factors include prolonged neutropenia, use of high-dose steroids or immunosuppressive agents, and conditions leading to impaired cellular immune responses such as human immunodeficiency virus infection or hematologic malignancies.123
IA typically involves the lungs, but may also infect the nasal sinuses, central nervous system, and rarely gastrointestinal (GI) system.134 GI aspergillosis is usually the result of organ involvement from disseminated systemic infection; isolated GI aspergillosis is rare even in immunocompromised hosts. This case shows that IA should be considered in critically ill patients without classical risk factors for timely and appropriate management.
A 47-year-old man visited the emergency room because of decreased mentality. He was diagnosed with diabetes mellitus (DM), but had not received regular treatment. His blood pressure was 60/30 mm Hg and body temperature was 38.5℃. Laboratory data showed white blood cell of 13.150×109/L, with a differential count of 90.7% neutrophils and 8.5% lymphocytes, hemoglobin of 14.3 g/dL, platelet count of 507×109/L, glucose levels of 443 mg/dL, blood urea nitrogen of 52.5 mg/dL, serum creatinine concentrations of 1.9 mg/dL, aspartate aminotransferase of 69 IU/L, alanine transaminase of 38 IU/L, sodium of 125.7 mmol/L, potassium of 5.4 mmol/L, C-reactive protein (CRP) levels of 19.42 mg/dL, ketone bodies in the blood 3 positive and HbA1c of 18.20%. Arterial blood gas analysis revealed a pH of 7.032, pCO2 of 21.8 mm Hg, pO2 of 83.3 mm Hg, and HCO3 of 5.8 mmol/L. Chest X-ray and computed tomography (CT) scans showed multiple consolidation and ground glass opacities in both lung fields (Fig. 1A and B). The patient was initially diagnosed with diabetic ketoacidosis as a result of severe pneumonia. An immediate hydration and insulin therapy were started, and dose-adjusted piperacillin and tazobactam sodium were also administered for pneumonia. The patient was admitted to the intensive care unit (ICU) for mechanical ventilator therapy. The ketoacidosis had improved with hydration and insulin therapy, but on the second day of hospitalization, diuretic-resistant pulmonary edema developed and continuous renal replacement therapy was started. Klebsiella pneumonia was identified in sputum cultures. After 8 days of treatment, the lung lesions had regressed (Fig. 1C). The patient was finally moved to the general ward. However, on the 5th day in the general ward, he started complaining of vague abdominal pain. His pain had aggravated with severe tenderness. An erect abdominal X-ray showed gaseous distention of small bowel loops with suspicious stepladder sign, suggesting mechanical obstruction (Fig. 2A). Subsequent abdominal CT suggested multiple perforation of the transverse colon with panperitonitis (Fig. 2B and C).
An emergency laparotomy was performed. Necrotic intestines were observed from the distal ascending colon to the proximal transverse colon. Necrotic portions were resected and the Periodic Acid Schiff and Grocott's methenamine silver staining of the resected specimen showed septated fungal hyphae with acute angle branching, suggesting aspergillus species (Fig. 3). The patient was finally diagnosed with colonic IA. Intravenous liposomal amphotericin-B (3 mg/kg/day) was additionally administered for 35 days. He was discharged with oral voriconazole to treat his reported remaining, abdominal discomfort and mildly elevated CRP levels. Voriconazole was discontinued at a follow-up visit to the outpatient department 2 weeks after discharge when his abdominal discomfort had relieved and CRP levels normalized.
When inhaled, aspergillus spores can cause upper respiratory tract and alveoli infections manifesting as pneumonia.1 Aspergillus spores are ingested and can reach the upper GI tract, but cannot penetrate the normal intact mucosal barrier. However, they can penetrate the mucosal barrier in pathologic conditions such as gastric ulcers and severe gastritis. In these situations, these spores can cause invasive gastric or upper GI aspergillosis.4
There are only a few studies regarding GI aspergillosis in immunocompromised hosts. The largest study by Kazan, et al.5 investigated 21 cases of primary and disseminated GI aspergillosis and showed that clinical manifestations of GI aspergillosis are nonspecific, such as abdominal pain, diarrhea, hemorrhage, and occasionally intestinal obstruction and perforation. This study concluded that invasive GI aspergillosis was rare and its diagnosis extremely challenging without surgical investigation due to poor symptom specificity and the absence of characteristic image findings. IA is rarely considered until characteristic fungal hyphae are observed during pathological investigation. Diagnosis can be made by culturing aspergillus, observing tissue invasion by aspergillus hyphae, or destruction and mucosal changes in tissue biopsy specimens.
Meersseman, et al.6 reported that considerable numbers of ICU patients without underlying hematologic diseases are diagnosed with IA. They suggested that some factors such as prolonged use of antibiotics, use of central venous catheters, and mechanical ventilation in the ICU may adversely affect the immune systems of critically ill patients and that patients in sepsis with multi-organ failure have decreased immunity because sepsis causes biphasic immunologic patterns.7 The initial phase is hyperinflammation, which is later counterbalanced by a following anti-inflammatory response or relative immune paralysis phase characterized by monocyte deactivation and associated with increased risk of opportunistic infections such as invasive pulmonary aspergillosis in ICU patients.
It is also well known that hyperglycemia in DM impairs immune responses including phagocytosis and complement function, which may permit bacterial or fungal colonization of skin or mucosa and may sometimes be associated with systemic infections.8910
Choi, et al.11 described a lower GI aspergillosis case in the non-neutropenic host. However, colon cancer, steroid use for 16-days and DM may be associated with immune dysfunction in their patient. Our case is different from the case described by Choi, et al.; our patient did not have cancer and steroid use, but was diagnosed with diabetic ketoacidosis at the initial presentation.
To our knowledge, there have been no reports on non-classical risk factors for GI aspergillosis. However, ICU care, antibiotic use, and mechanical ventilation in our patient could be equivalent to previously reported non-classical risk factors for IA in critically ill patients.6 All of these factors and uncontrolled DM appeared to contribute to invasive GI aspergillosis in this patient.4
We suggest that IA should be considered in critically ill patients with non-classical risk factors for timely diagnosis and appropriate treatment because it is no longer only a disease of immunocompromised hosts. In addition, primary or isolated lower GI aspergillosis may also occur in the immunocompetent hosts without classical risk factors.
Figures and Tables
Fig. 1
(A) Chest X-ray showing multifocal patchy pneumonic consolidation in both lungs. (B) Chest computed tomography showing extensive multifocal ground glass opacities accompanying consolidation, suggestive of pneumonia with acute respiratory distress syndrome. (C) Chest X-ray shows regression of pneumonic consolidation on the 9th day of admission.
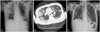
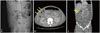
Fig. 2
(A) Erect abdominal X-ray showing gaseous distention of small bowel loops with an absence of colonic gas, suggestive of mechanical obstruction. (B and C) Enhanced abdominal computed tomography scan showing multifocal wall disruption (arrows) of the hepatic flexure of the ascending colon and proximal transverse colon with large amount of ascites and peritoneal thickening, suggestive of pan-peritonitis.
References
1. Segal BH. Aspergillosis. N Engl J Med. 2009; 360:1870–1884.
2. Maertens J, Vrebos M, Boogaerts M. Assessing risk factors for systemic fungal infections. Eur J Cancer Care (Engl). 2001; 10:56–62.
3. Stevens DA, Melikian GL. Aspergillosis in the 'nonimmunocompromised' host. Immunol Invest. 2011; 40:751–766.

4. Eggimann P, Chevrolet JC, Starobinski M, Majno P, Totsch M, Chapuis B, et al. Primary invasive aspergillosis of the digestive tract: report of two cases and review of the literature. Infection. 2006; 34:333–338.

5. Kazan E, Maertens J, Herbrecht R, Weisser M, Gachot B, Vekhoff A, et al. A retrospective series of gut aspergillosis in haematology patients. Clin Microbiol Infect. 2011; 17:588–594.

6. Meersseman W, Lagrou K, Maertens J, Van Wijngaerden E. Invasive aspergillosis in the intensive care unit. Clin Infect Dis. 2007; 45:205–216.
7. Kox WJ, Volk T, Kox SN, Volk HD. Immunomodulatory therapies in sepsis. Intensive Care Med. 2000; 26:Suppl 1. S124–S128.
8. Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997; 14:29–34.

9. Hostetter MK. Handicaps to host defense. Effects of hyperglycemia on C3 and Candida albicans. Diabetes. 1990; 39:271–275.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download