This article has been corrected. See "Erratum to "Snakebite in Korea: A Guideline to Primary Surgical Management" by Rha JH, et al. (Yonsei Med J 2015;56:1443-8.)" in Volume 57 on page 1050.
Abstract
Purpose
Snakebite is an emergency which causes local symptoms such as pain and edema around the bite. Systemic symptoms may also develop, such as dizziness or renal failure, and may even cause death. The purpose of this research was to assess the validity and safety of snakebite protocol for surgery when treating snakebite patients.
Materials and Methods
Retrospective research was performed on patients who were admitted after being treated at the emergency center from January 2008 to December 2012. When necessary, debridement was also performed, and 46 of 111 patients (41.4%) underwent debridement. Those who had received debridement without antivenom administration due to a positive skin reaction test were classified as group A, and group B received antivenom and delayed debridement. We reviewed the emergency and admission charts of the patients in each group and recorded and compared their age, sex, bite site, severity of local and general symptoms, time to receive antivenin, and complications.
Results
Of the ten patients (21.7%) in group A, two (66.6%) developed cellulites, and one of them experienced skin necrosis, resulting in a skin graft. In group B, there were 36 patients (78.2%), 19 (52.7%) of whom developed cellulitis. Skin necrosis occurred in two patients, and one of these patients received a skin graft. Compartment syndrome was found in one patient, and fasciotomy and a skin graft were performed.
Venomous snakes have specialized teeth (fangs) that release venom from venom glands connected to the fangs. Approximately 10% of 3500 species of venomous snakes are known to be harmful to humans, and an estimated 9000 injuries due to snakebite are reported to occur in the United States annually.12 According to a report by the World Health Organization, an estimated 300000 snakebite injuries occur annually, among which mortality occurs in 30000 to 40000 cases. Southeast Asia reported the highest mortality rate due to snakebite injury with 25000 deaths.3 There are four species of venomous snakes residing in Korea: Gloydius brevicaudus, Gloydius saxatilis, Gloydius ussuriensis, and Rhabdophi tigrinus. Their venoms are not very toxic, and systemic symptoms resulting from these snakes are rare; consequently, there are few mortality cases reported.4 While no accurate statistics on snakebite are available in Korea, a substantial number of snakebite injuries may occur annually. According to the Fire Department of Gangwon Province, approximately 30 snake appearances have been reported, and venomous snakebite injury occurred in a water park located in Daegu in June 2013. There was also a sighting of venomous snakes in the downtown area of Seoul in 2012. Our hospital has dealt with approximately 20 cases of venomous snakebite in the emergency room, indicating that it is not a rare injury. However, there is no central theory that acts as a standard for the treatment of venomous snakebite, and the guidelines for antivenom injection or dosage vary among hospitals as well. Most studies are case reports or complication reports, and research data on the use of antivenom and surgical management according to Korean snake species and severity of symptoms are lacking, such that the current practice is based on data reported from outside the country. The author retrospectively studied 111 patients who visited Wonju Severance Christian Hospital from January 2008 to December 2012 for venomous snakebite. Antivenom administration according to snake species, toxicity severity, and surgical severity of the wound was analyzed, and the safety and value of surgical management were investigated.
The medical records of 111 patients who visited Wonju Severance Christian Hospital from January 2008 to December 2012 for the management of venomous snakebite wounds were retrospectively reviewed, and 46 patients who required surgical management were included. To eliminate cases in which the cause of the bite wound was unclear, cases were only included if the wound site showed two fang marks. The following exclusion criteria were also used in the study: refusal to be monitored for 6 hours with a severity of Grade I or lower, refusal to be admitted for a severity of Grade II or higher, previous history of coagulation difficulty, age of 18 years or younger, history of administration of antivenom injection in another hospital, follow-up loss, and refusal to be included in the study.
The guidelines for the management of snakebite were developed based on the author's experiences and on published studies (both domestic and abroad) (Fig. 1). Vital signs of patients were checked upon arrival to the emergency room, and the species of the responsible snake was confirmed during history-taking. The severity of the bite wound was evaluated primarily to determine the administration of antivenom, and laboratory tests including complete blood count, general chemistry, coagulation profile, and creatinine kinase were performed to discover and manage systemic symptoms or treatments.
The classification proposed by Parrish, McCollough, and Gennard (Table 1),5 which was based on clinical findings and blood laboratory testing, was used to evaluate the severity of the bite wound and to determine the use of antivenom. The wound was irrigated with normal saline and then soaked with Betadine solution in cases in which antivenom was not provided. Antivenom was routinely administered to patients with a severity of Grade II or higher regardless of whether the venom source was confirmed or unknown. Antivenom was also provided regardless of patient severity in cases of swelling, erythema, and severe pain involving two or more joint surfaces adjacent to the bite wound. The decision to administer antivenom was also made after clinical reassessments, including checking blood laboratory tests (same as above) every 6 hours, checking vital signs every 30 minutes, and reexamining the wound. A skin reaction test was performed prior to antivenom administration in order to prevent anaphylactic shock or serum sickness. The skin test was performed with a 1-mL mixture of antivenom powder effective against A. brevicaudus (KoVax Dried Antivenom Injection, Korea Vaccine) and sodium chloride solution, which was then mixed with 9 mL normal saline. Then, 0.1 mL of the solution was injected subcutaneously, and the antivenom was discontinued in cases of positive results including erythema or swelling greater than 1 cm and shock. In cases of equivocal results, the remaining 0.9 mL of the solution was administered subcutaneously, and patients were assessed for a positive reaction after 30 minutes. The initial dose of antivenom was 6000 IU in cases of Grade II injury and 12000 IU in cases of more severe injury. A booster dose of 50% of the initial dose was provided if there was no clinical improvement within 12 hours after the initial administration or in the following cases: elevated PT/PTT, INR greater than 2, increased subcutaneous hemorrhage at the bite wound site, decreased blood pressure, or altered mental state. Antihistamine (pheniramine 4 mg) and hydrocortisone (Solu-Cortef 100 mg) were also provided to protect against complications associated with antivenom use (anaphylactic shock, decreased blood pressure, pulmonary edema, decreased cardiac output). All patients were also vaccinated against tetanus and prophylactic antibiotics (Quinolone). In cases in which antivenom was not provided, debridement of the wound followed by saline irrigation and Betadine soaking was immediately performed. If antivenom was provided, delayed debridement was performed 72 hours after the injury, and wound soaking was performed twice daily with acidic water made using a medical ionic-water generator (Dion Magic, Shenpix, Japan). Debridement was performed to remove as much of the necrotized tissue as possible until healthy tissue was revealed.6 Grade 0 and I patients were discharged after confirming the absence of clinical worsening (wound site pain, swelling progressing, etc.) during 6 hours of observation and normal blood laboratory test results. Patients with a severity of Grade II or higher were admitted. In cases in which surgical intervention was provided, follow-up outpatient visits were scheduled for 7 and 28 days after discharge.
Debridement was required in 46 of 111 patients (41.4%) with snakebite. Patients who skipped antivenom and underwent immediate debridement due to positive skin reaction against antivenom or due to injury caused by the Rhabdophisgenus (no antivenom exists for this genus) were classified as group A (10 patients, 21.7%), and patients who underwent delayed debridement after receiving antivenom were classified as group B (36 patients, 78.2%). The disease severity, mean age, past history, wound location, blood laboratory test results, timing of debridement, type of surgical intervention, and complications were compared between groups. Complications resulting from antivenom administration, including anaphylactic shock and decreased blood pressure, were also studied.
Statistical analysis was performed with SPSS version 13.0 (IBM Corp., Chicago, IL, USA), and the statistical difference between the two groups was determined with the Mann-Whitney U test or chi-square test. Statistical analysis involving stratification of severity was performed using the Kruskal-Wallis test, and Fisher's exact test was used for frequencies of less than 5. p<0.05 was set as statistically significant.
A total of 46 patients with snakebite who required surgical management were included. The mean age was 52.3±14.8 years, and there were 32 males and 14 females. The male-to-female ratio was approximately 2.3:1. The mean time to the emergency room was 122±184 minutes, and the mean duration of hospital stay was 8.4±2.3 days. There was no significant difference in injury grade. The most frequent site of injury included the fingers and hand (30 patients, 65.2%) (Table 2).
In group A, Grade I comprised the largest portion of patients (6, 60%). Group B included 20 patients (55.5%) with Grade II disease, suggesting relatively higher severity; however, the difference was not statistically significant. Age, sex, and past history were also similar between the two groups. The hand was the most frequent injury site for both groups, and no significant difference in injury site distribution was noted (Table 3).
In group A, two of three Grade II patients developed cellulitis, and a skin graft under general anesthesia was performed for a single case due to skin necrosis. In group B, cellulitis developed in nine patients, and two of them underwent a skin graft due to skin necrosis. Also, one Grade III patient in group B developed compartment syndrome, and fasciotomy under general anesthesia was performed. The wound was sutured 2 weeks later; however, partial skin necrosis developed, for which a skin graft was performed.
There were no surgical complications in either group that resulted in permanent functional disability, such as amputation, permanent joint contraction, neurologic damage, or osteomyelitis. A skin rash was found on one patient in group B and was treated with antihistamine.
There were one and four incidences of rhabdomyolysis in group A (10.0%) and group B (11.1%), respectively. Disseminated intravascular coagulation occurred in two patients in group A (20.0%) and seven patients in group B (19.4%). Progression to acute renal failure occurred in one case in group A (10.0%) and three cases in group B (8.3%). All complications were treated at the early phase, and there were no incidences of severe complication or mortality (Table 4).
A total of 16 species of snakes are known to reside in Korea, of which only four are poisonous: three species of the genus Gloydius (G.b. brevicaudus, G. saxatilis, G. ussuriensis) and one species of the genus Rhabdophis (R. tigrinus).7 Studies so far have reported G. ussuriensis to be the most common cause of snakebite due to its large population and easily accessible habitation area at the foot of mountains or in farm fields. There are no accurate statistics on the frequency of venomous snakebite injuries in Korea, although an average of 409.6 patients are reported to visit the hospital annually, which may increase when including patients with minor injuries who do not visit the hospital.8 Although reports vary on the gender ratio of patients with snakebite injuries, males are more common by an approximately 3:1 ratio, and the majority of the literature has found those in their sixth decade of life to be most frequently injured.9 The hands and feet, especially the distal areas such as the fingers and toes, were the predominant sites of injury, with the lower extremities being more frequently injured, unlike other countries.5 While several studies have reported a lack of association between location of snake bite, severity, and treatment results,10 these studies have mainly focused only on medical complications, and investigations on the results of surgical treatments are still lacking. Most such studies have focused on the muscle and the skin, as the lower extremities are the main bite injury sites abroad. In Korea, however, the hands are frequently targeted; therefore, studies investigating the effects of snake venom on ligaments and nerves are necessary. Patients with snakebite injuries develop a wide variety of clinical courses, from mild symptoms such as pain and edema at the injury site to serious complications including acute kidney failure, acute respiratory failure, myocardial infarction, hemoptysis, disseminated intravascular coagulation, coma, and death.11
From a surgical perspective, the first dilemma the clinician faces on managing a snakebite wound is determining whether to perform debridement on the wound site. In principle, trauma patients should undergo debridement as soon as possible, thereby removing the necrotized tissue in order to prevent further infection. In the past, early removal of snake venom by surgical methods were recommended as immediate management.121314151617 Due to fear of complications caused by antivenoms, several authors in the past have performed wide debridement as soon as possible, followed by application of tourniquets or ice bags while omitting the use of antivenoms.1819 However, the majority of such treatments resulted in soft tissue damage, leading to failed skin graft or flap, amputation, and osteomyelitis, ultimately leading to a poor prognosis. Immediate debridement is no longer the treatment of choice in managing snakebites; rather, the current standard of care is administration of antivenom followed by delayed debridement.20212223242526 A recent study using an electron microscope observed viable tissues buried inside necrotized muscles of the snakebite wound, providing additional evidence for preemptive antivenom use followed by delayed debridement. Nonetheless, these are findings from studies performed outside of Korea; domestic studies regarding this subject are few, and there is no standardized treatment guideline on surgical management of venomous snakebite.
Methods involving animals, mainly injecting toxins (IgG) in horses, have been frequently used in manufacturing antivenoms. This was also the case in Korea until 2002. However, the use of all IgG antibodies from the animal has resulted in frequent complications, such as anaphylactic shock; therefore, Crotalidae Polyvalent Immune Fab, which utilizes the binding site of immunoglobulin with the snake toxin, was developed in 2002, and it has received approval from the Korea Food and Drug Administration. According to meta-analyses, clinical results after the use of Fab in snakebite wounds were excellent with few severe adverse effects; thus, IgG-based antivenom is no longer used.22 The amount of antivenom serum required for treating venomous snakebites from Korean species is much less than that needed for snakebites from American species, and the incidence of serum reaction and hypersensitivity is even less. This finding was the basis for performing this study: should patients showing positive skin reaction to antivenoms receive antivenom and delayed debridement or simply immediate debridement? This study found that good clinical results may be expected even after adjusting the timing of debridement according to the use of antivenom. However, this finding may also be interpreted as a benign clinical course of snakebites caused by Korean species regardless of the effects of antivenom, which acts as a limitation of this study. The decision to administer antivenom based on the species of the venomous snake is questionable. The genus Rhabdophis is classified as a venomous snake in Korea, and its venom does result in clinical symptoms. However, the antivenom produced in Korea is only effective on venoms produced by the Agkistrodon genus. If an accurate distinction of the snakes can be achieved, unnecessary use of antivenom may be reduced, and debridement may be performed immediately; such distinction may help establish the priority of snakebite management, as well as save excessive medical expenses.
The limitations of this study are as follows. First, this study included only the results of a single emergency medical center and was based on a retrospective review of medical records, limiting its application to a wider population. Second, the sample size was small (particularly the number of critical patients), and grade IV patients could not be included in this study. In order to overcome these limitations, an additional prospective multi-institutional study investigating the appropriate management of venomous snakebite is necessary.
The decision to administer antivenom according to pre-established protocol, to perform debridement on injured soft tissue, and to provide secondary treatment to the affected area achieved relatively good clinical results. The guidelines suggested by this study may be useful in managing patients with venomous snakebite injuries.
Figures and Tables
Table 1
Grade of Envenomation7
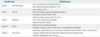
Table 2
Basal Characteristics of Patients

Table 3
Comparison between Groups A and B

References
1. Parrish HM. Incidence of treated snakebites in the United States. Public Health Rep. 1966; 81:269–276.
2. Warrell DA. Venomous bites and stings in the tropical world. Med J Aust. 1993; 159:773–779.
3. Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake bite in South Asia: a review. PLoS Negl Trop Dis. 2010; 4:e603.

4. Jang IS, Lee JA, Kim SY, Hyun SC, Park SM, Park JS, et al. Clinical features in snake bite. J Korean Soc Emerg Med. 1996; 7:580–589.
5. Shin CS, Bae JS, Sohn KS. Clinical Analysis on Venomous Snake Bite in Korea. J Korean Surg Soc. 1984; 27:245–254.
6. Toschlog EA, Bauer CR, Hall EL, Dart RC, Khatri V, Lavonas EJ. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013; 217:726–735.

7. Lee BJ, Hong SI, Kim HS, Kim TH, Lee JH, Kim HJ, et al. Hematological features of coagulopathy and the efficacy of antivenin therapy for a Korean snakebite. J Korean Surg Soc. 2007; 72:18–26.
8. Shim JH, Son YJ, Lee SS, Park KS, Oh HB, Park YD. Ecological study on poisonous snake and investigation of the venom characteristics, snakebiting frequency in Korea. Korean J Env Eco. 1998; 12:58–77.
9. Oh SJ, Yoo IH, Kim JP. A clinical review of venomous snake bite. J Korean Surg Soc. 1982; 24:1236–1244.
10. Kim ES, Choi WJ. Clinical review of venomous snake bite. J Korean Surg Soc. 2000; 59:433–440.
12. Glass TG Jr. Early debridement in pit viper bite. Surg Gynecol Obstet. 1973; 136:774–776.
13. Huang TT, Lynch JB, Larson DL, Lewis SR. The use of excisional therapy in the management of snakebite. Ann Surg. 1974; 179:598–607.

16. Huang TT, Blackwell SJ, Lewis SR. Hand deformities in patients with snakebite. Plast Reconstr Surg. 1978; 62:32–36.

17. Huang TT, Blackwell SJ, Lewis SR. Tissue necrosis in snakebite. Tex Med. 1981; 77:53–58.
18. Cohen WR, Wetzel W, Kadish A. Local heat and cold application after eastern cottonmouth moccasin (Agkistrodon piscivorus) envenomation in the rat: effect on tissue injury. Toxicon. 1992; 30:1383–1386.

19. Frank HA. Snakebite or frostbite: what are we doing? An evaluation of cryotherapy for envenomation. Calif Med. 1971; 114:25–27.
20. Lavonas EJ, Kokko J, Schaeffer TH, Mlynarchek SL, Bogdan GM, Dart RC. Short-term outcomes after Fab antivenom therapy for severe crotaline snakebite. Ann Emerg Med. 2011; 57:128–137.

21. Dart RC, Seifert SA, Boyer LV, Clark RF, Hall E, McKinney P, et al. A randomized multicenter trial of crotalinae polyvalent immune Fab (ovine) antivenom for the treatment for crotaline snakebite in the United States. Arch Intern Med. 2001; 161:2030–2036.

22. Yin S, Kokko J, Lavonas E, Mlynarchek S, Bogdan G, Schaeffer T. Factors associated with difficulty achieving initial control with crotalidae polyvalent immune fab antivenom in snakebite patients. Acad Emerg Med. 2011; 18:46–52.

23. Dart RC, Seifert SA, Carroll L, Clark RF, Hall E, Boyer-Hassen LV, et al. Affinity-purified, mixed monospecific crotalid antivenom ovine Fab for the treatment of crotalid venom poisoning. Ann Emerg Med. 1997; 30:33–39.

24. Lavonas EJ, Gerardo CJ, O'Malley G, Arnold TC, Bush SP, Banner W Jr, et al. Initial experience with Crotalidae polyvalent immune Fab (ovine) antivenom in the treatment of copperhead snakebite. Ann Emerg Med. 2004; 43:200–206.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download