Abstract
Purpose
Postoperative nausea and vomiting (PONV) is a common problem after general anesthesia. Although 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists have significantly reduced PONV, over 35% of patients treated with ondansetron can experience PONV. In this study, we investigated whether the Y129S and -100_-102AAG deletion polymorphisms of the 5-HT3B receptor gene affect the efficacy of ondansetron in preventing PONV.
Materials and Methods
Two hundred and forty-five adult patients who underwent laparoscopic cholecystectomy were enrolled. Ondansetron 0.1 mg/kg was intravenously administered 30 minutes before the end of surgery. Genomic DNA was prepared from blood samples using a nucleic acid isolation device. Both the Y129S variant and the -100_-102AAG deletion variant were screened for using a single base primer extension assay and a DNA direct sequencing method, respectively. The relationship between genetic polymorphisms and clinical outcomes of ondansetron treatment was investigated.
Results
Among the 5-HT3B AAG deletion genotypes, the incidence of PONV was higher in patients with the homomutant than with other genotypes during the first 2 hours after surgery (p=0.02). There were no significant differences in the incidence of PONV among genotypes at 2-24 hours after surgery. In the Y129S variants of the 5-HT3B receptor gene, there were no significant differences in the incidence of PONV among genotypes during the first 2 hours and at 2-24 hours after surgery.
Postoperative nausea and vomiting (PONV) is a common and unpleasant experience for surgical patients undergoing general anesthesia.12 The incidence of PONV is approximately 20-30%, although it can be as high as 80% in patients with risk factors.1 PONV may result in adverse events such as prolongation of the recovery period, delay of discharge, and postsurgical complications, and as such, it should be prevented by adequate management.34
Selective 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists have been widely researched and are currently used as the primary therapy for PONV prevention, as they have fewer side-effects such as sedation or extrapyramidal symptoms than other antiemetics.567 Although 5-HT3 receptor antagonists have significantly reduced PONV, it is reported that over 35% of patients treated with ondansetron experience PONV.3 One reason for this unsatisfactory response may be individual differences in the biotransformation and disposition of 5-HT3 receptor antagonists due to gene polymorphisms related to pharmacokinetics.13 However, the variations in gene coding for the 5-HT3 receptor as a target structure of the drug may also affect the extent of PONV and the antiemetic effects of 5-HT3 receptor antagonists.891011
In previous pharmacogenomic studies, Y129S and -100_ -102AAG deletion variants of the 5-HT3B receptor gene were found to be possibly associated with nausea induced by paroxetine and the clinical response of 5-HT3 receptor antagonists for chemotherapy-induced nausea and vomiting (CINV), respectively.9101213 We thus hypothesized that these polymorphisms might have a relationship with the therapeutic failure of 5-HT3 receptor antagonists for PONV treatment. In this study, we investigated whether the Y129S and -100_-102AAG deletion polymorphisms as variants of the 5-HT3B receptor gene affect the efficacy of ondansetron in preventing PONV in patients undergoing general anesthesia for laparoscopic surgery.
After obtaining Institutional Review Board approval and written informed consent from all patients, 288 adult patients of the American Society of Anesthesiologists (ASA) physical status I or II and ages 18 to 65 years who were scheduled to undergo laparoscopic cholecystectomy were recruited in this study. Before the surgery, all patients were interviewed to determine their past medical history, including tobacco and alcohol use, the presence of previous postoperative nausea or vomiting, and motion sickness. Patients with a history of drug abuse, a known prolongation of the QTc interval on electrocardiography, morbid obesity (body mass index >31 kg/m2), antiemetics use within 24 hours before the studyction of anesthesia, the patients' lungs were ventilated with 50% oxygen. Anesthesia, and significant hepatic or renal disease were excluded from the study.
Patients did not receive premedication. Upon arrival in the operating room, all patients were monitored with electrocardiography, noninvasive blood pressure, and pulse oximetry. Anesthesia was induced with thiopental 4-5 mg/kg, remifentanil 1.0 µg/kg, and rocuronium 0.6 mg/kg. After indu was maintained with sevoflurane 1.5-2.0% and remifentanil 0.1-0.2 µg/kg/min. Patients were kept warm using a forced-air warming system (Bair-Hugger®, Augustine-Medical, Eden Prairie, MN, USA) to maintain a body temperature of 36.0-37.0℃. Bispectral index (BIS) was measured continuously on a monitor (A-2000 BIS monitor, Aspect Medical Systems, Norwood, MA, USA) to maintain appropriate anesthetic depth throughout the operation. Thirty minutes before the end of surgery, ondansetron 0.1 mg/kg was administered intravenously, and ketorolac 60 mg was given for postoperative pain management. When the patients were able to obey commands, they were extubated. The total dose of remifentanil during anesthesia, and the pain score (0=no pain, 10=most extensive pain) upon arrival in the post anesthesia care unit were assessed.
The intensity of PONV was assessed during the first 2 hours after surgery and at 2-24 hours after surgery using a 10-cm visual analogue scale (0=no nausea, 10=most extensive nausea). At the same time, patients were monitored to determine whether an episode of vomiting occurred and whether antiemetics were administered. Episodes of retching were considered to be vomiting. During each time period, the maximal degree of PONV experienced by the patient was recorded, and any episodes of nausea, retching or vomiting were defined as the presence of PONV. If a patient experienced no episodes of nausea or vomiting after the administration of ondansetron, he or she was allocated to the no-PONV group. When the patient vomited or requested a rescue antiemetic, metoclopramide 10 mg was administered intravenously. Patients who reported a pain score >4 or requested rescue analgesics were treated with intramuscular injection of tramadol 50 mg. The primary endpoint of this study was the proportion of patients who did not experience any episodes of PONV after the administration of ondansetron for the prevention of PONV for each genotype group.
Genomic DNA was prepared from blood samples using a nucleic acid isolation device, QuickGene-mini80 (FUJIFILM, Tokyo, Japan). Genotyping for Y129S variants of the 5-HT3B receptor gene was screened through a single base primer extension assay using the ABI PRISM SNaPshot Multiplex kit (Applied Biosystem, Foster City, CA, USA) according to the manufacturer's recommendations. Analysis was performed using GeneMapper software (version 3.0; Applied Biosystems). Genotyping of the -100_-102AAG deletion variants of the 5-HT3B receptor gene was carried out using a DNA direct sequencing method. PCR was used to amplify one 5-HT3B fragment using UCSC In-Silico PCR. The PCR products were purified using a Multi-Screen384-PCR Filter Plate (Millipore, Billerica, MA, USA). The purified products were then sequenced using a BigDye Terminator Cycle Sequencing Kit and an ABI 3730xl automated sequencer (Applied Biosystems). Mutation analyses were performed using the Phred, Phrap, Consed, and Polyphred 5.04 software packages (University of Washington, Seattle, WA, USA).
Considering that the expected frequency of the AAG deletion allele was 16.5% based on a previous study,14 we estimated that to achieve a power of 90% (α=0.05, β=0.1) when assuming a difference of 20% in the failure rate for ondansetron treatment among the genotypes, 240 patients would be required. Therefore, we recruited 288 patients to compensate for excluded patients. The frequencies of the single nucleotide polymorphisms (SNPs) were assessed for deviation from Hardy-Weinberg equilibrium using Fisher's exact test. Frequency differences in genotype, demographic data, severity of PONV, and incidence of PONV were compared by the chi-square test, Fisher's exact test, the Kruskal-Wallis test, or an ANOVA with Bonferroni correction as appropriate. Data were analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). A value of p<0.05 was considered significant.
Of the 288 patients assessed to participate in this study, 43 were excluded from the study due to refusal to participate or sampling error. Ultimately, a total of 245 patients were enrolled. There were no significant differences in patient characteristics and clinical data among genotypes (Table 1 and 2).
The frequencies of all genotypes were in Hardy-Weinberg equilibrium (p>0.05). Frequencies of the 5-HT3B AAG deletion genotypes were as follows: 77.6% for wild type, 19.6% for heteromutant, and 2.8% for homomutant. Among the 5-HT3B AAG deletion genotypes, the incidence of PONV was higher in patients with a homomutant genotype than other genotypes during the first 2 hours after surgery (Codominant model, p=0.02) (Table 3). This difference was also statistically significant when analyzed between the patients with and without the Ins/ins (Dominant model, p=0.04) or the Del/del genotype (Recessive model, p=0.02), respectively. There were no significant differences in the incidence of PONV among genotypes at 2-24 hours after surgery. In the Y129S variants of the 5-HT3B receptor gene, there were no significant differences in the incidence of PONV among genotypes during the first 2 hours or at 2-24 hours after surgery (Table 4).
The severity of nausea was significantly higher in patients with a homomutant genotype than other genotypes during the first 2 hours after surgery in the 5-HT3B AAG deletion genotypes (p=0.02) (Table 5). There were no significant differences in the severity of nausea among genotypes in the Y129S variants of the 5-HT3B receptor gene (Table 6).
The present study revealed a significant association between the -100_-102AAG deletion polymorphism and the efficacy of ondansetron. The patients with a homomutant genotype of the -100_-102AAG deletion variant had a higher incidence of PONV than other genotypes during the first 2 hours after surgery; however, the Y129S genotypes were not related to the clinical response to ondansetron.
The 5-HT3 receptor antagonists have been used effectively in order to prevent PONV.57 Unfortunately, all patients do not show a favorable outcome when the drug is administered.3 This therapeutic failure may be partially explained by other complex mechanisms of PONV, such as the effects of histamine, dopamine, and opioids other than 5-HT3.115 Considering the increased occurrence of PONV in several generations of the same family, considerable concordance of PONV in twins, and the established high risk of PONV in children with a family history of PONV, the individual variability of drug responses can also be associated with inherited differences in genes modifying the drug's actions.10 This variability in drug action may be pharmacokinetic or pharmacodynamic.8 Genetic variations affecting the efficacy of 5-HT3 receptor antagonists have also been studied from a pharmacokinetic and pharmacodynamic perspective.101316
The primary target in pharmacokinetic investigations includes the CYP450 2D6 isoform (CYP2D6) associated with metabolic pathways of 5-HT3 receptor antagonists. Polymorphic variations of the CYP2D6 system may induce ultra-rapid metabolism and subsequent therapeutic failure of 5-HT3 receptor antagonists.10
Variations in genes encoding the target molecule of a drug as pharmacodynamic factors are associated with an alteration of drug activity.8 As the primary target of 5-HT3 receptor antagonists, the 5-HT3 receptor is a ligand-operated ion channel that includes five different receptor subunits (5-HT3A, B, C, D, and E) identified in humans.817 The 5-HT3A subunits are capable of forming a functional homomeric complex of the 5-HT3 receptor, whereas the 5-HT3B subunit forms a heteromeric complex through an association with the 5-HT3A subunit.816 The primary differences between the homomeric 5-HT3A and heteromeric 5-HT3A/3B complexes are biologic characteristics, conductance, and other slight differences in their pharmacological properties.817 The function and expression of the 5-HT3 receptor can be affected through variation of the genes coding its subunits; in particular, polymorphisms of the 5-HT3B subunit gene can modify the pharmacological and functional properties of the 5-HT3 receptor.1718 The Y129S and -100_-102AAG deletions investigated in this study are the typical genetic variations of the 5-HT3B subunit.91016 Y129S is a substitution of an amino-acid located in the coding region of the 5-HT3B subunit gene. Sugai, et al.12 demonstrated that the Y129S polymorphism significantly affected the incidence of nausea induced by paroxetine; however, in a previous study on the efficacy of 5-HT3 receptor antagonists in CINV, the Y129S polymorphism did not change the incidence of CINV.13 In this study, the Y129S polymorphism also did not change the response to ondansetron for PONV. Although this inconsistency may be explained by differences in factors such as paroxetine use, emetogenic chemotherapy administration, and general anesthesia, the reason for the inconsistent results is not clear.12 The -100_-102AAG deletion is a 3-base pair deletion within the 5'untranslated region of the 5-HT3B subunit gene.10 Tremblay, et al.13 demonstrated that this deletion variant in the promoter region was significantly associated with a high incidence of CINV, whereas Rueffert, et al.9 did not confirm this specific result in patients with PONV. The present study showed that patients who were homomutant for the AAG deletion genotype had a higher incidence of PONV than other genotypes. The discrepancy between Rueffert's and our results may have been caused by differences in patient characteristics and study design.9
Despite our favorable result, the functional impact of the -100_-102AAG deletion on the 5-HT3B receptor subunit is not yet obvious, and an in vitro study on the functional aspects of polymorphisms related to the 5-HT3B receptor subunit is still lacking.13 Furthermore, various issues related to the interactions existing between the subunits that make up the 5-HT3 receptor have not been fully explained, even though the 5-HT3B receptor subunit is likely to play an important functional role, considering the current knowledge about the 5-HT3 receptor.18 However, a previous study using secondary structure prediction showed that this polymorphism can cause a structural modification of mRNA when compared with the wild type.19 Meineke, et al.20 demonstrated that the -100_-102AAG deletion increased the promoter activity of the 5-HT3B receptor gene in vitro. In addition, this variant is a deletion of three nucleotides. Therefore, it could have a much greater impact on the receptor function than other single nucleotide polyporphisms.13
We found that the response to ondansetron for PONV did not differ according to the 5-HT3B -100_-102AAG deletion genotypes at 2-24 hours after surgery; however, in this study, 89% or more patients did not suffer from PONV between 2 and 24 hours, which may explain the lack of association observed during this time period.
There are a variety of risk factors that can affect the development of PONV. It is well-documented that female gender, a previous history of PONV or motion sickness, the use of intravenous opioids, and non-smoking status are the main predictors of PONV.1521 In this study, we did not find any significant differences in these predictors based on genotype. In order to minimize the influence of anesthetic and surgical factors on our results, we enrolled patients who underwent an identical surgical procedure and performed general anesthesia using the same anesthetic agent while maintaining BIS values at a constant level. Furthermore, our study confirmed that genotype distribution of the Y129S SNP and -100_-102AAG deletion of the 5-HT3B receptor gene was in accordance with Hardy-Weinberg equilibrium, suggesting that our finding involving this receptor gene is likely robust.
We concluded that the response to ondansetron for PONV was significantly influenced by the -100_-102AAG deletion polymorphisms of the 5-HT3B gene. Taking these results into account, the -100_-102AAG deletion variants may be a phar-macogenetic predictor of responsiveness to ondansetron for PONV. Further research is needed to clarify the functional role of this polymorphism in the 5-HT3B subunit and the interaction between the 5-HT3 receptor subunits.
Figures and Tables
Table 1
Patients and Clinical Characteristics of the Patients According to 5-HT3B AAG Deletion Genotype

Table 2
Patients and Clinical Characteristics of the Patients According to Y129S Variants of the 5-HT3B Receptor Gene
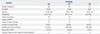
Table 3
Effects of 5-HT3B AAG Deletion Genotype on the Efficacy of Ondansetron for Postoperative Nausea and Vomiting
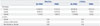
Table 4
Effects of Y129S Variants of the 5-HT3B Receptor Gene on the Efficacy of Ondansetron for Postoperative Nausea and Vomiting
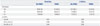
Table 5
Effects of 5-HT3B AAG Deletion Genotype on the Efficacy of Ondansetron for Nausea Severity

| First 2 hrs | 2-24 hrs | |
|---|---|---|
| Genotypes | ||
| Ins/ins | 0 (0-3) | 0 (0-0) |
| Ins/del | 0 (0-4) | 0 (0-0) |
| Del/del | 4 (2-5) | 0 (0-0) |
| p values | ||
| Overall | 0.03 | 0.43 |
| Non-Del/del vs. Del/del | 0.02 | 0.33 |
| Ins/ins vs. Non-Ins/ins | 0.05 | 0.26 |
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (No. 2010-0004868).
This study was registered with Clinicaltrials.gov (NCT0165 7786).
References
1. Candiotti KA, Birnbach DJ, Lubarsky DA, Nhuch F, Kamat A, Koch WH, et al. The impact of pharmacogenomics on postoperative nausea and vomiting: do CYP2D6 allele copy number and polymorphisms affect the success or failure of ondansetron prophylaxis? Anesthesiology. 2005; 102:543–549.
2. Lunn DV, Lauder GR, Williams AR, Pickering RM, McQuillian PJ. Low-dose droperidol reduces postoperative vomiting in paediatric day surgery. Br J Anaesth. 1995; 74:509–511.

3. Choi EM, Lee MG, Lee SH, Choi KW, Choi SH. Association of ABCB1 polymorphisms with the efficacy of ondansetron for postoperative nausea and vomiting. Anaesthesia. 2010; 65:996–1000.

4. White H, Black RJ, Jones M, Mar Fan GC. Randomized comparison of two anti-emetic strategies in high-risk patients undergoing day-case gynaecological surgery. Br J Anaesth. 2007; 98:470–476.

5. Bunce KT, Tyers MB. The role of 5-HT in postoperative nausea and vomiting. Br J Anaesth. 1992; 69:7 Suppl 1. 60S–62S.

6. Moon YE, Joo J, Kim JE, Lee Y. Anti-emetic effect of ondansetron and palonosetron in thyroidectomy: a prospective, randomized, double-blind study. Br J Anaesth. 2012; 108:417–422.
7. Kim SI, Kim SC, Baek YH, Ok SY, Kim SH. Comparison of ramosetron with ondansetron for prevention of postoperative nausea and vomiting in patients undergoing gynaecological surgery. Br J Anaesth. 2009; 103:549–553.

8. Kaiser R, Tremblay PB, Sezer O, Possinger K, Roots I, Brockmöller J. Investigation of the association between 5-HT3A receptor gene polymorphisms and efficiency of antiemetic treatment with 5-HT3 receptor antagonists. Pharmacogenetics. 2004; 14:271–278.

9. Rueffert H, Thieme V, Wallenborn J, Lemnitz N, Bergmann A, Rudlof K, et al. Do variations in the 5-HT3A and 5-HT3B serotonin receptor genes (HTR3A and HTR3B) influence the occurrence of postoperative vomiting? Anesth Analg. 2009; 109:1442–1447.

10. Janicki PK, Sugino S. Genetic factors associated with pharmacotherapy and background sensitivity to postoperative and chemotherapy-induced nausea and vomiting. Exp Brain Res. 2014; 232:2613–2625.

11. Fasching PA, Kollmannsberger B, Strissel PL, Niesler B, Engel J, Kreis H, et al. Polymorphisms in the novel serotonin receptor subunit gene HTR3C show different risks for acute chemotherapy-induced vomiting after anthracycline chemotherapy. J Cancer Res Clin Oncol. 2008; 134:1079–1086.

12. Sugai T, Suzuki Y, Sawamura K, Fukui N, Inoue Y, Someya T. The effect of 5-hydroxytryptamine 3A and 3B receptor genes on nausea induced by paroxetine. Pharmacogenomics J. 2006; 6:351–356.
13. Tremblay PB, Kaiser R, Sezer O, Rosler N, Schelenz C, Possinger K, et al. Variations in the 5-hydroxytryptamine type 3B receptor gene as predictors of the efficacy of antiemetic treatment in cancer patients. J Clin Oncol. 2003; 21:2147–2155.

14. Kato M, Fukuda T, Wakeno M, Fukuda K, Okugawa G, Ikenaga Y, et al. Effects of the serotonin type 2A, 3A and 3B receptor and the serotonin transporter genes on paroxetine and fluvoxamine efficacy and adverse drug reactions in depressed Japanese patients. Neuropsychobiology. 2006; 53:186–195.
15. Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992; 77:162–184.
16. Krzywkowski K. Do polymorphisms in the human 5-HT3 genes contribute to pathological phenotypes? Biochem Soc Trans. 2006; 34(Pt 5):872–876.

17. Dubin AE, Huvar R, D'Andrea MR, Pyati J, Zhu JY, Joy KC, et al. The pharmacological and functional characteristics of the serotonin 5-HT(3A) receptor are specifically modified by a 5-HT(3B) receptor subunit. J Biol Chem. 1999; 274:30799–30810.
18. Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, et al. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999; 397:359–363.

19. Frank B, Niesler B, Nöthen MM, Neidt H, Propping P, Bondy B, et al. Investigation of the human serotonin receptor gene HTR3B in bipolar affective and schizophrenic patients. Am J Med Genet B Neuropsychiatr Genet. 2004; 131B:1–5.
20. Meineke C, Tzvetkov MV, Bokelmann K, Oetjen E, Hirsch-Ernst K, Kaiser R, et al. Functional characterization of a -100_-102delAAG deletion-insertion polymorphism in the promoter region of the HTR3B gene. Pharmacogenet Genomics. 2008; 18:219–230.

21. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999; 91:693–700.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download