Abstract
Purpose
To report clinical characteristics of thyroid-associated ophthalmopathy (TAO) in patients who previously underwent total thyroidectomy for thyroid cancer or a benign mass of the thyroid.
Materials and Methods
Of the patients who were diagnosed with TAO from March 2008 to March 2012, we performed a retrospective chart review on those who had undergone total thyroidectomy for thyroid cancer or a benign mass of the thyroid before the occurrence of ophthalmopathy.
Results
Of the 206 patients diagnosed with TAO, seven (3.4%) met the inclusion criteria. The mean age of the subjects was 47.4 years, and all were female. Six patients were diagnosed with papillary thyroid cancer, and one was diagnosed with a benign mass. The duration between total thyroidectomy and onset of TAO ranged from 3-120 months (median 48 months). Ophthalmic manifestations varied among cases. Except for the patient who was diagnosed with a benign mass, all patients showed hyperthyroid status and were under Synthroid hormone treatment at the time of TAO development. Five of these six patients had positive levels of thyroid-stimulating hormone (TSH) receptor autoantibodies.
Thyroid associated ophthalmopathy (TAO) is an autoimmune inflammatory orbital disorder most commonly associated with Graves' disease (GD).1234 According to Tanda, et al.,5 one-third of 346 GD patients at a single center present ophthalmic manifestation at their initial visit, and nearly 20% of patients who do not present Graves' ophthalmopathy (GO) at their initial visit develop ophthalmopathy during the follow-up period. Although the pathophysiology of this mechanism is not fully understood, the thyroid-stimulating hormone (TSH) receptor, which contains thyroid follicular cells and orbital connective tissue, might act as a common autoantigen.126 TSH receptor autoantibodies have been associated with the severity or activity of GO.789 Recently, several studies regarding the role of T helper 1- (CXCL 10) and T helper 2- (CCL2) chemokine in GO pathogenesis were introduced.34
In terms of the association between TAO, GD, and thyroid cancer, patients with GD have a higher incidence of papillary thyroid cancer (PTC) than those without GD, which may be due to the higher thyroid hormone activity in GD patients than in the normal population.10 However, the development of hyperthyroid GD in patients with thyroid cancer is rare.1112 In 1997, Kasuga, et al.12 showed that out of 1680 partial thyroidectomies performed from 1966 to 1993 to remove thyroid nodules, only four GD cases (0.24%) were reported. Given that TAO is closely associated with hyperthyroidism, the development of TAO in patients diagnosed with thyroid cancer is presumably rare.
In the present study, we investigated the clinical characteristics of TAO in patients who underwent total thyroidectomy for non-GD cases (i.e., thyroid cancer or a benign mass).
We retrospectively reviewed the medical records of TAO patients who visited the Ophthalmology Department at Severance Hospital between March 2008 and March 2012 as well as selected patients who had undergone total thyroidectomy for thyroid nodules or cancer before the development of ophthalmopathy. Patients who had previous abnormal thyroid function, a history of GD, or any signs or symptoms of TAO prior to the thyroid operation were excluded from this study.
Diagnosis of TAO was made by one ophthalmology clinician (JSY) based on the following criteria: eyelid retraction, proptosis, extraocular muscle involvement, motility restriction, computed tomography findings, and/or eyelid swelling.
Age, sex, treatment after total thyroidectomy, duration between total thyroidectomy and radioiodine (RAI) therapy, and duration between the surgery and ocular symptoms were reported. Thyroid cancer staging was assessed based on the 2002 American Joint Committee on Cancer tumor node metastasis criteria.
All laboratory data at the time of ophthalmopathy occurrence were reviewed. Levels of 3,5,3'-triiodothyronine (T3), thyroxine (T4), free T4, TSH, thyroglobulin (Tg), anti-peroxidase antibodies (antiTPO), and TSH receptor antibodies, including thyroid-binding inhibitory immunoglobulin (TBII) and thyroid-stimulating immunoglobulin (TSI), were investigated.
Of the 206 patients diagnosed with TAO, seven (3.4%) met the inclusion criteria. The mean age of the subjects was 47.4±8.1 years, and all were female. Six patients were diagnosed with PTC, and one was diagnosed with a benign thyroid mass. Of the six patients diagnosed with PTC, two presented with stage I cancer, and four presented with stage III cancer. These four patients underwent a thyroid scan uptake test, and all showed positive findings. RAI treatment was performed on these four patients using a dose of 30 mCi (Table 1).
The duration between total thyroidectomy and onset of TAO ranged from 3-120 months (median 48 months). The ophthalmic manifestations varied among cases, with active TAO [clinical activity score (CAS)≥3] diagnosed in four patients (cases 1, 2, 6, 7), proptosis recorded in five patients (cases 1, 2, 4, 5, 6), and extraocular muscle limitation identified in two patients (cases 6, 7). One patient (case 3) presented with unilateral upper eyelid retraction only (Table 1, Fig. 1).
Except for one patient who was in the euthyroid state and diagnosed with a benign thyroid mass, all other patients were in the hyperthyroid state under Synthroid treatment. Five of the six patients who underwent the TSH receptor autoantibody test showed positive findings. Laboratory and clinical manifestations were summarized in Table 2.
A 51-year-old woman presented with left upper eyelid retraction and left eye proptosis (Fig. 1A). Twenty-seven months prior to the first visit to the ophthalmology department, she underwent total thyroidectomy for PTC stage III. RAI (30 mCi) was performed 2 months after the surgery, after which she began taking Synthroid oral pills until her visit to the ophthalmologic department. At the time of the visit, TSH was suppressed (less than 0.025 mIU/L), and the free T4 level was slightly elevated (1.9 ng/dL). TBII and TSI were 2.38 IU/L and 441.4 percentage of specimen-to-reference ratio (SRR%), respectively. PET scan revealed no recurrence of thyroid cancer, and Tg was within normal limits (10.9 IU/mL). The CAS for TAO was 3, and triamcinolone was injected for the treatment of upper eyelid retraction.
A 49-year-old woman presented with right upper eyelid retraction and right eye proptosis (Fig. 1B). She underwent total thyroidectomy for stage III PTC, and RAI was performed 1 month postoperatively. Four months later, she visited the ophthalmologic department with ocular symptoms. The CAS was 3, and a 3-mm right eye proptosis was identified. She was taking 0.15 mg Synthroid. At the time of her visit, TSH was suppressed (0.14 mIU/L), and the free T4 level was slightly elevated (2.53 ng/dL). TBII and TSI were not measured. Mullerectomy was performed for the treatment of upper eyelid retraction.
A 47-year-old woman presented with right upper eyelid retraction (Fig. 1C). She underwent total thyroidectomy for stage III PTC, and RAI was performed 1 month later. After 3 months of RAI, she visited the ophthalmologic department with newly developed right upper eyelid retraction yet without any other specific findings. The patient was taking 0.15 mg Synthroid, and her thyroid function test revealed hyperthyroid status (TSH 0.12 mIU/L, free T4 2.03 ng/dL). TBII was elevated (1.79 IU/L).
A 55-year-old woman presented with both upper eyelid retraction and proptosis (Fig. 1D). Three months prior to her visit, she underwent total thyroidectomy for stage III PTC. RAI was performed 1 month later. TBII was elevated (4.82 IU/L). The patient was taking 0.075 mg Synthroid, and a thyroid function test revealed hyperthyroid status (TSH 0.4 mIU/L, free T4 1.65 ng/dL). Tg was within normal limits (116.1 IU/mL).
A 58-year-old woman presented with a 4-mm right eye proptosis (Fig. 1E). Her medical history revealed that she had undergone total thyroidectomy for PTC stage I yet did not receive RAI therapy. She was taking 0.1 mg Synthroid, and her thyroid function test revealed hyperthyroid status (TSH <0.025 mIU/L, free T4 1.76 ng/dL). TBII and TSI bioassays were both within normal limits (0.38 IU/L and 53.9 SRR%, respectively), and both Tg (16.3 IU/mL) and antiTPO (10.5 IU/mL) were within normal limits.
A 39-year-old woman presented with both ocular pain and swelling. She had undergone total thyroidectomy for a benign thyroid mass 10 years before her visit. She was not taking Synthroid, and her thyroid function was normal (TSH 0.22 mIU/L, free T4 1.31 ng/dL). Ophthalmic examination showed that CAS was 7, indicating severe active TAO. Upper eyelid retraction and a 24-mm proptosis were present in both eyes (Fig. 1F), as was diplopia in primary gaze. TBII (over 40 IU/L) and TSI (676 SRR%) were significantly elevated; however, Tg was within normal limits (47.2 IU/mL). She was given systemic corticosteroid treatment for active TAO.
A 44-year-old woman presented with a 3-month history of painless diplopia (Fig. 1G). She underwent near-total thyroidectomy for PTC stage I. Ocular motility revealed limitation of supraduction with right eye hypotropia. Upper eyelid retraction was present in both eyes, and the CAS was 3. Tg (330.1 IU/mL) and the antiTPO level (447.4 IU/mL) were both elevated. She was taking 0.1 mg Synthroid. TSH was suppressed (less than 0.025 mIU/L), and the free T4 level was slightly elevated (1.67 ng/dL). TBII and TSI were 4.82 IU/L and 716 SRR%, respectively. She was treated with right inferior rectus muscle recession for right hypotropia.
We reported seven rare cases of patients who developed TAO after total thyroidectomy for thyroid cancer or a benign mass of the thyroid. Interestingly, most patients showed abnormalities in thyroid function and/or TSH receptor autoantibody tests at the time of TAO onset. Menconi, et al.13 compared overall improvement of Graves' orbitopathy between near-total thyroidectomy and total thyroid ablation groups, reporting better outcomes of orbitopathy in the total thyroidectomy group. Winsa, et al.14 compared TSH receptor autoantibody levels and eye involvement between subtotal thyroidectomy and total thyroidectomy and found that those characteristics benefitted from total rather than subtotal thyroidectomy. These reports support the hypothesis that minimizing the remnant thyroid tissue may be beneficial for eye involvement through the removal of shared antigens and autoreactive T-lymphocytes.
Although the mechanisms through which TAO developed in these patients were unclear, we suggest several possibilities. In cases of euthyroid status, TAO development may be related to the presence of thyroid tissue that remains after total thyroidectomy (with or without RAI). RAI and even the thyroidectomy surgery itself may also contribute to ophthalmopathy.
We assumed that cases 1, 5, 6, and 7 might be euthyroid TAO cases following total thyroidectomy. Thyroid tissue may have remained even after total thyroidectomy had been performed. Yoon, et al.15 performed a study on five TAO patients with thyroid cancer who tested positive for TBII yet showed normal thyroid function, suggesting that TAO may develop due to systemic autoimmunity and may not be induced by hyperthyroidism. Case reports of euthyroid TAO several decades ago also describe patients with ocular signs of TAO and normal thyroid function without previous history of hyperthyroidism.1617 To our knowledge, our study is the first to report euthyroid TAO following total thyroidectomy. In the present study, case 6 demonstrated severe active TAO despite undergoing total thyroidectomy for a benign thyroid mass 10 years prior to the ophthalmology department visit. Thyroid function and Tg were all within normal limits at the time of TAO development; however, TBII and TSI titer were considerably elevated. As it was a benign mass, the patient did not receive RAI, which may have removed the remaining thyroid tissue. Therefore, we hypothesized that the remaining thyroid tissue may have induced abnormal thyroid autoimmunity even without any change in thyroid hormone. Following total thyroid ablation, a subsequent elevation of the serum Tg level is an indication of recurrence or metastasis of thyroid cancer. However, in case 7, the patient underwent near-total thyroidectomy for PTC stage I, and RAI was not performed. In this patient, the elevated Tg (330.1 IU/mL) level meant that there was certainly remaining thyroid tissue.
Of the six patients with PTC in the present study, four had stage III cancer. After total thyroidectomy and RAI, thyroid hormone was administered routinely to correct hypothyroidism and suppress serum TSH to less than 0.1 µU/mL, which inhibited the growth of remaining TSH-dependent thyroid cancer cells. In most of our cases, administrating thyroid hormone suppressed TSH and increased free T4. Although hyperthyroid status may not influence ophthalmopathy directly, it may have secondary effects on TAO through its negative influence of thyroid autoimmunity.
In three patients (cases 2, 3, and 4), TAO onset was closely related to RAI treatment after thyroidectomy. The relationship between RAI treatment and worsening of ophthalmopathy is well-documented.1819 Radiation injury may result in the release of the TSH receptor, which is a common antigen involved in GD and ophthalmopathy. Two unusual TAO cases have been reported recently. One involved a patient with metastatic thyroid cancer who developed TAO after RAI and external beam radiation.20 The other case involved a patient with disseminated thyroid cancer and no previous autoimmune thyroid disease who developed TAO after RAI treatment while under recombinant human thyrotropin and retinoic acid treatments, indicating that radiation-associated thyroid injury may contribute to TAO in patients with thyroid cancer.21
Considering that ophthalmopathy occurred 3-4 months after thyroidectomy in several of our cases, ophthalmopathy may be related to the thyroidectomy itself. Several previous cases have shown the development of GD shortly after thyroid cancer surgery.1112 Although the mechanism for GD development after partial thyroidectomy is unclear, several hypotheses have been suggested. The first hypothesis is that the operation destroys thyroid cells, which may increase TSH receptor expression and thereby lead to GD.12 A second hypothesis is that the operation causes postoperative GD by inducing an immune system abnormality, such as the stimulation of the antigen-presenting cells that control the activation of suppressor or regulatory cells. A third hypothesis is that the stress from general anesthesia and surgery affects the patients physically and mentally, thereby causing neuroendocrine fluctuations that disrupt immunological homeostasis. One final hypothesis is that postoperative bacterial and viral infections increase the number of CD5+ B cells, which stimulate the TSH receptor antibodies and thus contribute to GD.11
In conclusion, ophthalmopathy rarely develops in patients after total thyroidectomy for thyroid diseases other than GD, and the pathogenesis of TAO in these other thyroid diseases is unclear. However, thyroid hormonal function and TSH receptor antibody tests, which were conducted in most patients that showed ophthalmic symptoms, yielded abnormal results. Our results, combined with previous literature, indicated several possibilities for the pathogenesis of TAO. First, euthyroid TAO may be caused by thyroid tissues that remain after total thyroidectomy (with or without RAI). Second, RAI may contribute to the development of ophthalmopathy. Third, the surgery itself may lead to ophthalmopathy.
Figures and Tables
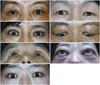 | Fig. 1(A) Case 1 shows left upper eyelid retraction. (B) Case 2 shows right upper eyelid retraction. (C) Case 3 shows right upper eyelid retraction. (D) Case 4 shows both upper eyelid retraction and eyelid swelling. (E) Case 5 shows right eye proptosis. (F) Case 6 shows eyelid swelling and erythema, both conjunctival injection and severe proptosis. (G) Case 7 shows right hypotropia. |
Table 1
Patients' General Characteristics

Table 2
Patients' Laboratory Characteristics

NC, not checked; N/A, not applicable; Tg, thyroid globulin; antiTPO, anti-thyroid peroxidase antibodies; TSH, thyroid stimulating hormone; TBII, thyroid-binding inhibitory immunoglobulin; TSI, thyroid-stimulating immunoglobulin; fT4, free thyroxine; SRR%, percentage of specimen-to-reference ratio.
Normal range: TBII 0-1.75 IU/L, TSI 0-140 SRR%, Tg Level 0-130.6 IU/mL, antiTPO 0-13.7 IU/mL, TSH 0.4-3 mIU/L, free T4 0.7-1.48 ng/dL.
ACKNOWLEDGEMENTS
This study was supported by Yonsei and Soonchunhyang University Research Fund. The funding organization had no role in the design or conduct of this research.
References
2. Kuriyan AE, Phipps RP, Feldon SE. The eye and thyroid disease. Curr Opin Ophthalmol. 2008; 19:499–506.

3. Antonelli A, Ferrari SM, Corrado A, Franceschini SS, Gelmini S, Ferrannini E, et al. Extra-ocular muscle cells from patients with Graves' ophthalmopathy secrete α (CXCL10) and β (CCL2) chemokines under the influence of cytokines that are modulated by PPARγ. Autoimmun Rev. 2014; 13:1160–1166.

4. Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. 2014; 13:272–280.

5. Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, et al. Prevalence and natural history of Graves' orbitopathy in a large series of patients with newly diagnosed graves' hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013; 98:1443–1449.

6. Lehmann GM, Feldon SE, Smith TJ, Phipps RP. Immune mechanisms in thyroid eye disease. Thyroid. 2008; 18:959–965.

7. Jang SY, Shin DY, Lee EJ, Yoon JS. Clinical characteristics of Graves' orbitopathy in patients showing discrepancy between levels from TBII assays and TSI bioassay. Clin Endocrinol (Oxf). 2014; 80:591–597.

8. Jang SY, Shin DY, Lee EJ, Choi YJ, Lee SY, Yoon JS. Correlation between TSH receptor antibody assays and clinical manifestations of Graves' orbitopathy. Yonsei Med J. 2013; 54:1033–1039.
9. Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves' orbitopathy. J Clin Endocrinol Metab. 2010; 95:2123–2131.

10. Carnell NE, Valente WA. Thyroid nodules in Graves' disease: classification, characterization, and response to treatment. Thyroid. 1998; 8:647–652.

11. Yu HM, Park SH, Lee JM, Park KS. Graves' Disease that Developed Shortly after Surgery for Thyroid Cancer. Endocrinol Metab (Seoul). 2013; 28:226–230.

12. Kasuga Y, Kobayashi S, Fujimori M, Shingu K, Hama Y, Ito K, et al. Development of Graves' disease after surgical treatment for thyroid nodules: report of four cases. Endocr J. 1997; 44:567–570.
13. Menconi F, Marinò M, Pinchera A, Rocchi R, Mazzi B, Nardi M, et al. Effects of total thyroid ablation versus near-total thyroidectomy alone on mild to moderate Graves' orbitopathy treated with intravenous glucocorticoids. J Clin Endocrinol Metab. 2007; 92:1653–1658.

14. Winsa B, Rastad J, Akerström G, Johansson H, Westermark K, Karlsson FA. Retrospective evaluation of subtotal and total thyroidectomy in Graves' disease with and without endocrine ophthalmopathy. Eur J Endocrinol. 1995; 132:406–412.

15. Yoon JS, Lew H, Park JS, Nam KH, Lee SY. Papillary thyroid carcinoma with thyroid-associated orbitopathy in a euthyroid state. Ophthal Plast Reconstr Surg. 2007; 23:187–191.
16. Eckstein AK, Lösch C, Glowacka D, Schott M, Mann K, Esser J, et al. Euthyroid and primarily hypothyroid patients develop milder and significantly more asymmetrical Graves ophthalmopathy. Br J Ophthalmol. 2009; 93:1052–1056.

17. Jang SY, Lee SY, Lee EJ, Yoon JS. Clinical features of thyroid-associated ophthalmopathy in clinically euthyroid Korean patients. Eye (Lond). 2012; 26:1263–1269.

18. Acharya SH, Avenell A, Philip S, Burr J, Bevan JS, Abraham P. Radioiodine therapy (RAI) for Graves' disease (GD) and the effect on ophthalmopathy: a systematic review. Clin Endocrinol (Oxf). 2008; 69:943–950.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download