Abstract
Purpose
Anastomotic airway complications are a major cause of morbidity and mortality after lung transplantation (LTx). In this study, the authors identified types and clinical outcomes of airway complications after LTx.
Materials and Methods
All bronchial anastomotic complications were analyzed in a total of 94 LTx cases involving 90 recipients who underwent surgery between July 2006 and May 2014. Fifteen LTx cases (14 recipients) with incomplete medical records for fiberoptic bronchoscopy (FBS) and three cases underwent heart-lung transplantation (HLT) were excluded. Postoperative FBS at 24-48 hours, 1, 3, 6, and 12 months, and then yearly after the transplantation were performed.
Results
A total of 76 LTx cases (75 recipients) were analyzed. The mean age of the recipients was 49.55 years (range, 18-71 years), and 38 (49.4%) were male. Twenty-one out of 76 cases (27.6%) experienced early anastomotic complications, and 12 (15.8%) presented late anastomotic complications. The early anastomotic airway complications presented in various forms: stenosis, 1 case; narrowing, 1; necrosis & dehiscence, 3; fistula, 4; granulation, 10; and infection, 2. Late complications almost entirely presented in the form of bronchial stenosis; five recipients showed stenosis at the anastomosis site, and one of them showed improvement after ballooning. Five others were found to have stenosis at the bronchus intermedius, distal to the anastomosis site. Three of these patients showed improvement after ballooning or bronchoplasty.
Since first lung transplantation (LTx) was performed in 1963 by James Hardy of the University of Mississippi in 1963, there has been significant progress in the surgical techniques and medical support used in this field. However, the median survival of LTx recipients is about 5.5 years, which is still disappointing compared to that of recipients of other solid organs.1 Following morbidity and mortality from infections, accounting for approximately 35% of deaths in the first year of LTx,2 airway complications are another major cause that deteriorates clinical outcomes of LTx recipients. The incidence of airway complications ranges broadly from 1.6% to 33% depending on the reporting system.3 In a large scale study, 18% of patients developed at least one anastomotic airway complication.4
In the early era of LTx, disruption of the airway anastomosis was one of major causes of death among patients who survived at least 2 weeks after transplantation. The procedure of LTx and the transplanted lung create a unique environment that is prone to anastomotic airway complications. In addition to the fact that the donor organ is devascularized and systemic arterial blood supply is not routinely connected, there are several unique factors that precipitate anastomotic airway complications, such as contamination of bronchial anastomoses fields, tension at the anastomosis site, and mismatched donor and recipient organ sizes.56
Anastomotic airway complications after LTx are classified according to either the time of development or the nature of structural deformities. Airway complications that develop within 3 months after surgery are designated as early complications whereas those after 3 months are late complications. Structural deformities at the bronchial anastomosis are classified as follows: stenosis, necrosis and dehiscence, exophytic granulation, bronchial malacia, fistula, and infection of the anastomosis.45 In the early era of LTx, bronchial complications such as dehiscence and necrosis were frequent causes of airway complications; these are classified as early complications.7 In recent years, the incidence of airway complications has decreased as a result of the improvement of surgical techniques and changes in presentation at the time of diagnosis.6 In a recent report by Santacruz and Mehta,3 necrosis and dehiscence was found to occur mainly in the early phase, while stenosis occurred mainly in the late phase. The incidence of early complications has been gradually declining, whereas the relative incidence of late complications, such as stenosis, has been increasing.
In this study, we reviewed anastomotic complications after LTx and classified them according to time of development. We also investigated underlying causes using the pathologic diagnosis, identifiable risks, procedures to overcome each complication, clinical outcome, and natural course of the morbidity. Identification of risk factors and better understanding of the natural course of anastomotic airway complications after LTx may lead to better clinical outcomes of recipients.
From July 1996, a total of 94 LTx cases in 90 recipients were performed as of May 2014 at Yonsei University Hospital. Of these cases, 15 (in 14 recipients) did not receive postoperative surveillance fiberoptic bronchoscopy (FBS) or had incomplete FBS medical reports, and three cases (three recipients) that involved heart-lung transplantation (HLT) were not analyzed in this study.
Demographic characteristics, primary lung diseases, microbiologic tests before and after transplantation, serial FBS, pulmonary function tests, and episodes of infection throughout the post-transplantation observation period were reviewed. This study was reviewed and approved by the Institutional Review Board at Gangnam Severance Hospital, Yonsei University College of Medicine (IRB No. #3-2012-0250).
Before transplantation, sputum and bronchoalveolar lavage fluids from recipients were collected, cultured, and analyzed for bacteria and fungus. Serologic tests for cytomegalovirus (CMV), Epstein-Barr virus, hepatitis A, B, and C, herpes virus, and human immunodeficiency virus were also included. Antimicrobial prophylaxis was based on the antibiotic sensitivity from the sputum culture of recipients and from the bronchial aspiration of donors. In patients whose colonization reports and antibiotic sensitivity were not available, a single agent, piperacillin-tazobactam, or a combination of ceftriaxone, isepamicin, and metronidazole were administered. Every patient underwent prophylaxis for fungal infection with fluconazole for one year and for Pneumocystis jirovecii (P. jirovecii) infection with sulfamethoxazole-trimethoprim. Patients with moderate or high risk for CMV infection, such as recipient (+)/donor (+ or -), and recipient (-)/donor (+), respectively, received prophylactic intravenous ganciclovir for two weeks, followed by oral valganciclovir for 6 months or up to one year. To induce immunosuppression, methylprednisolone was used followed by maintenance with steroids, calcineurin inhibitor, and anti-metabolites. Immunosuppression was maintained using medications consisting of prednisone, tacrolimus, and mycophenolate mofetil.
Post-transplantation evaluation included pulmonary function tests, chest imaging studies, sputum bacterial cultures, FBS, and CMV antigenemia testing. Postoperative FBS was performed at 24-48 hours, 1, 3, 6, and 12 months, and yearly after transplantation. For the evaluation of early and late airway complications, we diagnosed stenosis by visual assessment. Pulmonary function tests were performed at 1, 3, 6, and 12 months and yearly after transplantation. If respiratory infection or rejection was suspected, the patient underwent a chest CT scan, fibrobronchoscopy with alveolar lavage for bacterial and fungal cultures, indirect immunofluorescence tests for viral and P. jirovecii infections, mycobacterial polymerase chain reaction, and transbronchial biopsies.
A total of 94 LTx in 90 recipients who underwent surgery between July 2006 and May 2014 were collected, and 76 LTx cases (75 recipients) who had no identifiable problems during this time period underwent further analysis (Fig. 1). The mean age of recipients was 49.55 years (range, 18-71 years), and 38 (49. 4%) were male. Seven out of 76 underwent single LTx, and the remaining 69 cases were bilateral LTx. Twenty-four out of 76 (31.6%) were diagnosed as chronic obstructive pulmonary disease (COPD) using a pre-operative pulmonary function test. As LTx recipients showed obstructive patterns of flow-volume loop, indicating variant underlying causes such as bronchiectasis (non-cystic fibrosis), interstitial pulmonary fibrosis (IPF), or lymphangioleiomyomatosis (LAM), we classified subjects by pathological diagnosis. IPF was the leading cause of LTx, accounting for 36 cases, followed by LAM (13 cases), bronchiectasis (non-cystic fibrosis; eight cases), diffuse alveolar damage (four cases), diffuse panbronchiolitis (two cases), Eisenmenger syndrome (one case), interstitial lung disease related to connective tissue disease (four cases), bronchiolitis obliterance (BO) after bone marrow transplantation (BMT; five cases), and three other cases (Table 1). Twenty-one out of 76 recipients (27. 6%) experienced early anastomotic airway complications and 12 (15.8%) experienced late complications. Forty-four LTx cases (43 recipients) did not show complications at anastomosis sites during the follow-up period. In comparing recipients who suffered airway complications with those who did not, there were no statistical differences in age, gender, or ratio of donor-recipient total lung capacity. Recipients who suffered from IPF or bronchiectasis (non-cystic fibrosis) experienced airway complications more frequently than other disease subgroups. Among 33 LTx cases with anastomotic airway complications, one recipient with dehiscence died as a result of anastomotic airway complications.
Early anastomotic airway complications were defined as complications that developed within 3 months of LTx. Among 76 LTx recipients, 21 experienced early anastomotic airway complications, and the median time of detection for these complications was 26 days after transplantation. Early anastomotic complications presented in various forms: stenosis (one case), narrowing (one case), necrosis & dehiscence (three cases), fistula (four cases), granulation (ten cases), and infection (two cases) (Table 2). Stenosis was diagnosed by visual estimation. Five recipients, who experienced granulation and necrosis at anastomosis sites, respectively showed improvement with antibiotics and antiviral agents without endoscopic intervention. However, other forms of early anastomotic airway complications such as fistula, stenosis, and dehiscence required specific interventions. All four recipients who presented with a fistula 10-38 detection days after LTx underwent resuture or surgical repair, and three patients showed improvement on follow-up bronchoscopy. One recipient with a fistula that had developed at the proximal portion of the right upper lobe bronchus at postoperative day (POD) #12 underwent emergency open thoracotomy and surgical re-suture. After the re-suture, follow-up bronchoscopy revealed stenosis of the right upper lobe bronchus (Fig. 2A). Fungal infection at anastomosis sites was another form of early airway complication. Bronchoscopy, performed on an LTx recipient who experienced gradually aggravating dyspnea and rhonchi, revealed luminal narrowing with exophytic granulation tissues at both anastomosis sites. A biopsy was performed at the granulation and confirmed Aspergillus fumigatus infection. After treatment with antifungal agents, the granulation sites healed, and minimal stenosis developed as a sequela on a follow-up study after 2 months (Fig. 2B). Dehiscence at the anastomosis site was one of the most disastrous complications that developed without prodromal symptoms. One recipient experienced sudden and rapid clinical deterioration with shock. Emergency bronchoscopy was performed and dehiscence at both anastomosis sites was identified. Despite intensive medical care, the recipient died before surgical intervention was initiated.
Late anastomotic airway complications were defined as those developing at least 3 months after LTx, and most of them presented in the form of stenosis. Twelve recipients experienced late anastomotic airway complications, and the median time of airway complication was 179 days after LTx. Five recipients showed stenosis at anastomosis sites, and four presented stenosis at the bronchus intermedius, distal to the anastomosis site. One recipient diagnosed with BO after BMT presented focal stenosis at the right middle lobe. Among the nine recipients who showed stenosis at the right main bronchus (two cases), left main bronchus (two cases), both main bronchi (one case), and right bronchus intermedius (four cases), five recipients underwent balloon dilatation, and one recipient underwent surgical bronchoplasty (Table 3). Three out of five recipients who underwent bronchoscopic balloon dilatation showed improvement of airway stenosis. One patient who developed vanishing bronchus intermedius syndrome 295 days after LTx underwent surgical bronchoplasty, and the stenosis subsequently improved. Most cases required either surgical bronchoplasty or ballooning, except for one case that presented with stenosis at the left main bronchus and one case that presented with focal stenosis at the right middle lobe bronchus and was diagnosed with BO after BMT, which showed improvement without intervention. These two cases were treated only with antibiotics and antifungal agents in order to treat other forms of infection such as infectious colitis and pneumonia.
Among all LTx cases that presented with airway complications, only one case presented early airway complications in the form of fungal infection (case No. 6 in Table 2), and another single case that presented with persistent stenosis of both main bronchi and was classified as a late complication (case No. 32 in Table 3) was successfully treated with ballooning.
In the early era of LTx, anastomotic airway complications were one of the major factors that determined the clinical outcomes of LTx recipients.6 With risk-factor identification and the advancement of surgical techniques, the frequency and aspect of anastomotic airway complications has changed.
The prevalence of anastomotic airway complications varies widely depending on the reporting system. If all patents with abnormal findings by routine bronchoscopy were reported regardless of the need of intervention and associated symptoms, the prevalence of anastomotic airway complications is high, reaching up to 33%. On the other hand, if the reporting system counts only airway complications that are associated with symptoms, the prevalence of anastomotic complications is relatively low.5 Murthy, et al.4 indicated that only two thirds of patients with anastomotic complications have associated airway symptoms. In this study, the prevalence of anastomotic complications was 39.0%, due to serial routine bronchoscopy and an inclusive reporting system that counted cases with abnormal bronchoscopy findings regardless airway symptoms and intervention need.
Moreno, et al.6 reported that the risk factors for anastomotic complications include double LTx, post-transplantation airway colonization, and post-transplant intubation longer than 72 hours. In this study, stenosis was a frequent sequela after early complications, such as fistula and infection. Aside from a small number of cases, anastomotic complications occurred belatedly in the late complication group, which had no identifiable findings on serial bronchoscopy until at least 3 months after LTx, consequently making it difficult to identify the risk factors for anastomotic complications in this study. One patient with early anastomotic complications died, while the rests survived during the time of analysis. Anastomotic airway complications palliate several months after LTx; however, they influence the prognosis and late death of recipients.4 Furthermore, interventions on the complicated airway perpetuate and further deteriorate the condition, indicating a strong need of early identification in order to avoid the risks.5
Despite anastomotic airway complications, the majority of patients were able to maintain their lung function parameters over time. When the effect of anastomotic complications on the parameters from the pulmonary function test were investigated by serial-FEV1/preoperative-FEV1, there was no statistical difference in the change of lung function between pre-stenosis FEV1 and post-stenosis FEV1 when comparing those treated with ballooning or bronchoplasty, indicating that early and late anastomotic complications do not induce permanent deterioration of pulmonary lung function when proper intervention is provided (data not shown). The incidence of BO was two out of 32 recipients with anastomotic complications and three of out 50 without anastomotic complications, suggesting that BO might not be related to such complications.
In conclusion, the incidence of anastomotic airway complications was 39.0% based on routine serial bronchoscopy performed on LTx recipients who did not show postoperative complications. The complications were successfully managed by either surgical or endoscopic means and did not worsen the clinical outcomes of the LTx recipients.
Figures and Tables
 | Fig. 1Diagram of the study cases. Since July 1996, a total 90 patients (94 LTx cases) underwent LTx. Among 90 recipients, 18 LTx cases were excluded from this analysis. Among 76 LTx cases (75 recipients), 21 recipients showed early anastomotic complications and 12 late complications. LTx, lung transplantation; FOB, fiberoptic bronchoscopy; PFT, pulmonary function test. |
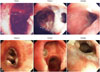 | Fig. 2Representative photographs of early anastomotic airway complications. (A) A case of early airway complication presenting fistula at anastomosis site. No specific findings at the anastomosis site were detected by immediate post-op bronchoscope. At POD#12, fistula at the proximal portion of the right upper lobe bronchus was detected and re-suture was performed. Follow-up bronchoscope performed at POD#90 revealed stenosis of right upper lobe bronchus. (B) Another case of early airway complication showing fungal infection at anastomotic sites. Exophytic granulation tissues with 30% luminal narrowing at both anastomosis sites were identified at POD#18. Bronchoscopic biopsy and culture study revealed the granulation from A. fumigatus infection. After treatment with antifungal agents, the granulations were disappeared but there were minimal stenosis on a follow-up study at POD#60. POD, postoperative day. |
Table 1
Demographic Characteristics of the Study Participants

SD, standard deviation; ILD, interstitial lung disease; BO after BMT, bronchiolitis obliterance after bone marrow transplantation; TLC, total lung capacity.
*p value was obtained from one-way analysis of variance (ANOVA), †p value was obtained from chi-square test, ‡p value was obtained from linear by linear association.
Table 2
Early Anastomotic Airway Complications

Table 3
Late Anastomotic Airway Complications

ACKNOWLEDGEMENTS
This study was supported by an institutional grant from Yonsei University College of Medicine (6-2011-0204) given to YS Chang.
References
1. Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. J Heart Lung Transplant. 2011; 30:1104–1122.

2. Aguilar-Guisado M, Givaldá J, Ussetti P, Ramos A, Morales P, Blanes M, et al. Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant. 2007; 7:1989–1996.

3. Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009; 6:79–93.

4. Murthy SC, Blackstone EH, Gildea TR, Gonzalez-Stawinski GV, Feng J, Budev M, et al. Impact of anastomotic airway complications after lung transplantation. Ann Thorac Surg. 2007; 84:401–409. 409.e1–409.e4.

5. Murthy SC, Gildea TR, Machuzak MS. Anastomotic airway complications after lung transplantation. Curr Opin Organ Transplant. 2010; 15:582–587.
6. Moreno P, Alvarez A, Algar FJ, Cano JR, Espinosa D, Cerezo F, et al. Incidence, management and clinical outcomes of patients with airway complications following lung transplantation. Eur J Cardiothorac Surg. 2008; 34:1198–1205.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download