Abstract
Purpose
Leptomeningeal collateral, in moyamoya disease (MMD), appears as an ivy sign on fluid-attenuated inversion-recovery (FLAIR) images. There has been little investigation into the relationship between presentation of ivy signs and old brain lesions. We aimed to evaluate clinical significance of ivy signs and whether they correlate with old brain lesions and the severity of clinical symptoms in patients with MMD.
Materials and Methods
FLAIR images of 83 patients were reviewed. Each cerebral hemisphere was divided into 4 regions and each region was scored based on the prominence of the ivy sign. Total ivy score (TIS) was defined as the sum of the scores from the eight regions and dominant hemispheric ivy sign (DHI) was determined by comparing the ivy scores from each hemisphere. According to the degree of ischemic symptoms, patients were classified into four subgroups: 1) nonspecific symptoms without motor weakness, 2) single transient ischemic attack (TIA), 3) recurrent TIA, or 4) complete stroke.
Results
TIS was significantly different as follows: 4.86±2.55 in patients with nonspecific symptoms, 5.89±3.10 in patients with single TIA, 9.60±3.98 in patients with recurrent TIA and 8.37±3.39 in patients with complete stroke (p=0.003). TIS associated with old lesions was significantly higher than those not associated with old lesions (9.35±4.22 vs. 7.49±3.37, p=0.032). We found a significant correlation between DHI and motor symptoms (p=0.001).
Moyamoya disease (MMD) is a chronic cerebrovascular occlusive disease with angiographic findings of bilateral internal carotid artery (ICA) terminal portion stenosis or occlusion.12 MMD has characteristic angiographic findings of abnormal vascular networks that can be seen in the vicinity of the occlusive arterial lesions and the prominent leptomeningeal and transdural collaterals. Leptomeningeal collateral flow appears as high signal intensity on fluid-attenuated inversion-recovery (FLAIR) images taken of the subarachnoid space; their characteristic look has led to them be called "ivy signs".3 Because ivy signs reflect the presence of leptomeningeal collaterals, not basal Moyamoya vessels, they can be seen in patients with unremarkable Moyamoya vessels, which typically show up as a flow void in the basal ganglia or basal cistern on T1- or T2-weighted images. Therefore, ivy signs can be useful for the diagnosis of MMD.4
Some studies have reported that the intensity of ivy signs indicates a negative relationship with cerebral vascular reserve (CVR)56 and a positive relationship with severity of ischemia.6 There have been few investigations into the relationship between ivy signs and old brain lesions, such as hemorrhagic or ischemic lesions. Silent microbleeds have been detected in approximately 15-44% of adult patients with MMD on T2*-weighted images,789 and about 20% of asymptomatic Moyamoya patients have been reported to have a silent cerebral infarction on brain MRI.10 The purpose of this study is to define the clinical significance of ivy signs, including their correlation with old brain lesions and the severity of clinical symptoms in patients with MMD.
After approval from the Institutional Review Board of our institute, we retrospectively reviewed the case histories of 83 patients with idiopathic MMD, who were diagnosed between February 2009 and May 2012. A total of 52 patients were female (mean age, 24.5±18.2 years; range, 3-63 years) and 31 were male (mean age, 19.7±17.4 years; range, 2-60 years). Diagnoses were established through clinical findings, conventional MRI and magnetic resonance angiography (MRA) according to the criteria established by the Research Committee on Spontaneous Occlusion of the Circle of Willis.11 We obtained clinical information by retrospective chart reviews.
The severity of the clinical symptoms was based specifically on the patients' motor symptoms. Patients were classified into four subgroups according to their frequency of motor weakness:612 1) nonspecific symptoms without motor weakness, 2) single transient ischemic attack (TIA), 3) recurrent TIA, and 4) complete stroke. Complete stroke included having an ischemic or hemorrhagic event that was confirmed through imaging. Patients with recurrent TIA and complete stroke were aggregated into a severe symptom group, and the patients with nonspecific symptoms or single TIA were classified as the mild symptom group.
MRI was performed with 3.0-T scanner (Achieva, Philips Medical Systems, Best, the Netherlands). FLAIR parameters for the scanner were set as follows: TR/TE=11000/125 ms, TI=2800 ms, FOV=23×23 cm, matrix size=352×238, slice thickness=5 mm, inter-slice gap=2 mm. The following were the T2* gradient echo (GRE) parameters: TR/TE=566/15 ms, matrix size=256×205. The DWI parameters were as follows: TR/TE=2048/45 ms; acquisition matrix size=128×125, with both b=0 and b=1000 s/mm2. Axial FLAIR images for 83 patients with MMD were independently reviewed and graded by two neuroradiologists who were blinded to any clinical information.
In this study, ivy sign on FLAIR images was defined as increased signal intensity in the subarachnoid space to reflect maximally dilated pial vasculature.6 In order to evaluate ivy signs in each cerebral hemisphere, each cerebral hemisphere was divided into 4 regions along the anterior to posterior direction; we set regional boundaries at the anterior cerebral artery territory, the anterior half of the middle cerebral artery (MCA) territory, the posterior half of the MCA territory and the posterior cerebral artery (PCA) territory.6 Each region was scored (0-3 point scale) based on the prominence of ivy sign (Figs. 1 and 2): a score of 0 indicated an absence of ivy sign in the defined region; a score of 1 indicated the presence of ivy sign in less than one-third of the defined region; a score of 2 indicated that more than one-third of the region had ivy sign; and a score of 3 indicated that more than two-thirds of the region had ivy sign. The total ivy score (TIS, range of 0 to 24) was defined as the sum of the eight region scores, and the dominant hemispheric ivy sign (DHI) indicates which hemisphere demonstrated more intense amount of ivy sign and was determined by comparing the ivy scores of each hemisphere.
Silent, old ischemic lesions were identified using T2-weighted and FLAIR images. Old parenchymal hemorrhages and silent microbleeds were also detected with T2* GRE images, and diffusion-weighted imaging was used in diagnosing acute cerebral infarction.
We performed all statistical analyses using SPSS version 20.0 for Windows (IBM, Chicago, IL, USA). Interobserver agreement for the presence of ivy score was assessed by the κ-value and the 95% confidence interval (CI). The Kruskal-Wallis test was performed to compare TISs between four different groups. The t-test was performed to compare TISs between severe and mild symptom groups and between patients with and without old lesions. Spearman correlation test was performed to investigate correlation between TIS, ivy score of motor symptom related hemisphere and four different groups. Ordinal regression analysis was performed to investigate the strongest predictor of the severity of motor symptom. The χ2 test was used to analyze the differences between old lesions after lateralizing clinical symptoms by DHI or patient subgroups. The level of statistical significance was set to p<0.05.
According to the patients' clinical data, seven patients had nonspecific symptoms without motor weakness, like headache or seizure. Nine patients experienced a transient motor weakness and 48 patients had more than one experience of transient motor weakness. Nineteen patients were diagnosed with complete ischemic or hemorrhagic stroke by imaging. Thirty patients received encephalo-duro-arterio-synangiosis (EDAS) procedure within 1 month after diagnosis.
The ivy sign was observed in all moyamoya patients. Interobserver agreement was moderate to substantial for each brain region (Table 1). The TIS for all four subgroups were as follows; nonspecific symptoms=4.86±2.55, single TIA=5.89±3.10, recurrent TIA=9.60±3.98, and complete stroke=8.37±3.39 (Fig. 3A). There was a significant difference in TIS among the four subgroups (p=0.003). The TIS of the mild symptom group was lower than that of the severe symptom group (5.44±2.83 vs. 9.25±3.84, p<0.001) (Fig. 3B). After excluding the patients with nonspecific symptoms and bilateral motor symptoms, we analyzed the ivy score of motor symptom-related hemisphere in patients with unilateral symptoms, separately. The ivy scores of the patients in each group, according to the order listed above, were as follows: 2.75±1.17, 5.37±2.43, and 4.53±2.38 (p=0.006). There was a correlation between TIS and severity of motor symptom (p=0.016), but not between ivy score of motor symptom-related hemisphere and severity of motor symptom (p=0.098). Statistical model based on TIS was suitable to describe the severity of motor symptom (beta coefficient=0.109, p=0.047) whereas ivy score of motor symptom-related hemisphere was not suitable (p=0.422) in the ordinal regression analysis.
Old ischemic and hemorrhagic lesions on MRI were detected in 46 patients; 41 lesions were unilateral and 5 were bilateral (Table 2). The TIS in patients with old lesions was higher than in those without old lesions (7.49±3.37 vs. 9.35±4.22, p=0.032). Old lesions were detected in 3 out of 7 (42.9%) patients with nonspecific symptoms, 4 out of 9 (44.4%) patients with single TIA, 26 out of 48 (54.2%) patients with recurrent TIA and 13 out of 19 (68.4%) patients with complete stroke. We found significant differences in neither old lesion presence among the four subgroups (p=0.529) nor between the mild and severe symptom groups (43.8% vs. 58.2%, p=0.403).
DHI was determined in sixty-three patients. Twenty patients had equivalent ivy scores for both hemispheres; thus, a DHI could not be determined. Among the 46 patients with old ischemic or hemorrhagic lesions on MRI, DHI could not be determined in seven patients. We found a weakly significant correlation between the side of the brain where the old lesion occurred and DHI (p=0.052) (Table 3).
Sixty-five patients had lateralizing motor symptoms and 40 of these had clinical symptoms on the opposite side from the DHI. Eleven patients had bilateral motor symptoms and 7 of them had equal ivy scores for each hemisphere. The side of the brain where motor symptoms occurred significantly correlated with DHI (p=0.001) (Table 4).
In this study, we demonstrated that the ivy sign as measured by TIS, correlated with clinical findings and previous brain lesions, and that TIS was also significantly correlated with clinical severity and reflected the progression of MMD. Unlike a previous study,6 we did not analyze the brain by dividing both sides of the hemisphere because definite diagnosis of MMD requires the presence of bilateral steno-occlusive lesions in the terminal ICAs. TIS correlated strongly with severity of motor symptom compared with the ivy score of motor symptom related hemisphere, because some patients had clinical symptoms on the same side as the DHI. Thus, we propose that the degree of progression in patients with MMD is evaluated more accurately by TIS, not by the ivy score of just one hemisphere.
The TIS was lower in the mild symptom group than in the severe symptom group and was also lower in patients without old lesions than in those with old lesions. Whereas, a previous study found that leptomeningeal collaterals are mainly developed from the PCA in the MMD, ivy score was found to be higher in the anterior MCA region.6 Ivy sign was considered to reflect dilated pial arteries compensating for the decreased CVR rather than the leptomeningeal collaterals.6 Because CVR is reduced with MMD progression, collateral circulation and fine pial vasculature dilatation are developed, and these changes are presented as ivy sign. Therefore, high TIS likely reflects advanced-stage MMD and it can be used as an imaging marker for increased stroke risk. A study of a normal elderly population confirmed that patients with silent brain infarctions had a higher risk of stroke.13 Furthermore, the presence of silent microbleeds was a significant predictor of subsequent hemorrhagic stroke in adult patients with MMD.9 Previous studies have found that patients with a history of previous stroke had a greater potential for developing cerebral lesions,913 and the present study also found higher TIS in patients with a history of cerebral lesions. Therefore, we suggest that the likelihood of stroke is relatively high in patients with high TIS.
DHI was determined in order to explore the relationship between the severity of ivy sign and clinical symptoms and the location of old lesions. In our study, 75.5% of patients with identifiable DHI also had clinical symptom related to DHI. We also found that ivy sign is correlated with clinical symptoms. Although the trend was not significant according to our criteria, we did find a relationship between the hemisphere in which the old lesion was located and DHI. It is likely that brain lesions appear in the hemisphere with advanced-stage MMD.
The ability to diagnose asymptomatic MMD has increased with the improvement of imaging technology and increased implementation of frequent examinations.14 We found that 8.4% of the patients in our study were diagnosed with MMD despite having only nonspecific symptoms, which is consistent with a previous study.15 However, our results, which found that 49.9% of patients had ischemic lesions, differed from findings of Kuroda, et al.,10 which reported a lower prevalence. It is quite possible that this is because Kuroda, et al. included only patients with asymptomatic MMD. Old hemorrhagic lesions were detected in 15.7% of participants in this study; this prevalence was similar to results from previous studies.789 We did not find a significant difference in the proportion of lesions between the mild symptom group and the severe symptom group, although old lesions were detected more often in the severe symptom group than in the mild group.
Our study has several limitations. First, we did not analyze the relevance of ivy score or CVR or angiographic findings of MRA. If we conducted these analyses, our results would be similar to those of previous studies. However, we did not conduct these analyses because this study's main focus was on FLAIR image data and its relevance to clinical findings. Second, we initially designed cross-sectional study, but could not analyze natural course of disease because of considerable patients receiving EDAS and lack of follow up imaging data.
In conclusion, ivy sign was detected in all patients with MMD. It was highly correlated with clinical findings, and those with higher TIS also had a tendency of more frequent and severe ischemic motor symptom. However, further evaluation of longitudinal data is needed for determining treatment options and predicting clinical outcomes.
Figures and Tables
Fig. 1
Fluid-attenuated inversion-recovery MRI of 11-year-old child with recurrent left side motor weakness. The ivy score of the right hemisphere is 3 in the anterior and posterior MCA territory, 2 in the ACA territory, and 1 in the PCA territory. The ivy score of the left hemisphere is 2 in the ACA and posterior MCA territory, and 1 in the anterior MCA and PCA territory. ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery.
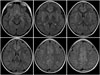
Fig. 2
Fluid-attenuated inversion-recovery MRI of 57-year-old adult with right side motor weakness due to ischemic stroke. The ivy scores of the both hemisphere are 1 in the anterior, posterior MCA and ACA territories, and 0 in the PCA territory. ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery.
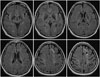
Fig. 3
Box-and-whisker plot showing total ivy score in patients classified according to the severity of clinical symptoms (A) and dichotomized by severity for each group of patients (B). TIA, transient ischemic attack.

Table 1
Ivy Score Kappa Values and 95% CIs

| Kappa (95% CI) | ||
|---|---|---|
| Right | Left | |
| ACA | 0.64 (0.50-0.77) | 0.57 (0.42-0.71) |
| Anterior MCA | 0.65 (053-0.77) | 0.55 (0.41-0.69) |
| Posterior MCA | 0.60 (0.47-0.73) | 0.72 (0.61-0.83) |
| PCA | 0.56 (0.42-0.69) | 0.62 (0.47-0.76) |
Table 2
Relationship between the Presence of an Old Lesion and Clinical Severity and Total Ivy Score

References
1. Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969; 20:288–299.
2. Takeuchi K, Shimizu K. Hypogenesis of bilateral internal carotid artery. Shinkei. 1957; 9:37–43.
3. Maeda M, Tsuchida C. "Ivy sign" on fluid-attenuated inversion-recovery images in childhood moyamoya disease. AJNR Am J Neuroradiol. 1999; 20:1836–1838.
4. Fujiwara H, Momoshima S, Kuribayashi S. Leptomeningeal high signal intensity (ivy sign) on fluid-attenuated inversion-recovery (FLAIR) MR images in moyamoya disease. Eur J Radiol. 2005; 55:224–230.

5. Kawashima M, Noguchi T, Takase Y, Ootsuka T, Kido N, Matsushima T. Unilateral hemispheric proliferation of ivy sign on fluid-attenuated inversion recovery images in moyamoya disease correlates highly with ipsilateral hemispheric decrease of cerebrovascular reserve. AJNR Am J Neuroradiol. 2009; 30:1709–1716.

6. Mori N, Mugikura S, Higano S, Kaneta T, Fujimura M, Umetsu A, et al. The leptomeningeal "ivy sign" on fluid-attenuated inversion recovery MR imaging in Moyamoya disease: a sign of decreased cerebral vascular reserve? AJNR Am J Neuroradiol. 2009; 30:930–935.

7. Ishikawa T, Kuroda S, Nakayama N, Terae S, Kudou K, Iwasaki Y. Prevalence of asymptomatic microbleeds in patients with moyamoya disease. Neurol Med Chir (Tokyo). 2005; 45:495–500.
8. Kikuta K, Takagi Y, Nozaki K, Hanakawa T, Okada T, Mikuni N, et al. Asymptomatic microbleeds in moyamoya disease: T2*-weighted gradient-echo magnetic resonance imaging study. J Neurosurg. 2005; 102:470–475.

9. Kuroda S, Kashiwazaki D, Ishikawa T, Nakayama N, Houkin K. Incidence, locations, and longitudinal course of silent microbleeds in moyamoya disease: a prospective T2*-weighted MRI study. Stroke. 2013; 44:516–518.
10. Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y. Research Committee on Moyamoya Disease in Japan. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke. 2007; 38:1430–1435.

11. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis. Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis. Neurol Med Chir (Tokyo). 2012; 52:245–266.
12. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis ('moyamoya' disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997; 99:Suppl 2. S238–S240.
13. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003; 34:1126–1129.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download