Abstract
Purpose
To evaluate the effect of bilateral subthalamic nucleus (STN) deep brain stimulation (DBS) on levodopa-induced peak-dose dyskinesia in patients with Parkinson's disease (PD).
Materials and Methods
A retrospective review was conducted on patients who underwent STN DBS for PD from May 2000 to July 2012. Only patients with levodopa-induced dyskinesia prior to surgery and more than 1 year of available follow-up data after DBS were included. The outcome measures included the dyskinesia subscore of the Unified Parkinson's Disease Rating Scale (UPDRS) part IV (items 32 to 34 of UPDRS part IV) and the levodopa equivalent daily dose (LEDD). The patients were divided into two groups based on preoperative to postoperative LEDD change at 12 months after the surgery: Group 1, LEDD decrease >15%; Group 2, all other patients. Group 2 was further divided by the location of DBS leads.
Results
Of the 100 patients enrolled, 67 were in Group 1, while those remaining were in Group 2. Twelve months after STN DBS, Groups 1 and 2 showed improvements of 61.90% and 57.14%, respectively, in the dyskinesia subscore. Group 1 was more likely to experience dyskinesia suppression; however, the association between the groups and dyskinesia suppression was not statistically significant (p=0.619). In Group 2, dyskinesia was significantly decreased by stimulation of the area above the STN in 18 patients compared to stimulation of the STN in 15 patients (p=0.048).
In 1995, the first patients with Parkinson's disease (PD) who underwent deep brain stimulation (DBS) of the bilateral subthalamic nucleus (STN) were described.1 Currently STN DBS is an accepted surgical treatment for the control of PD symptoms inadequately controlled by medical therapies.2345 Numerous studies, including randomized controlled trials, have demonstrated that this procedure can dramatically improve cardinal parkinsonian symptoms such as tremors, rigidity, and bradykinesia. It has also been demonstrated to improve levodopa-induced dyskinesia and reduce the required levodopa dosage for symptom control.2367 Levodopa-induced dyskinesia is a frequent and important cause of disability in PD and a major reason to recommend surgical treatment. In the literature, bilateral STN DBS is reported to cause a significant reduction of dyskinesia (60% to 80%) in most patients.89101112131415161718 Relief from dyskinesia after STN DBS has been hypothesized to be due to a postoperative reduction of dopaminergic medication;2719202122 however, some data suggest that STN DBS may also have direct dyskinesia-suppressing qualities. The objective of this study was to evaluate the effects of bilateral STN DBS on levodopa-induced dyskinesia in patients with PD after surgery, while taking into account levodopa dosage reductions.
We retrospectively reviewed the medical records of patients with PD who underwent bilateral STN DBS at our institution between May 2000 and July 2012. Patients with PD who suffered from severe levodopa-induced peak-dose dyskinesia before surgery were included. Patients who previously underwent thalamotomy or pallidotomy, which may suppress dyskinesia, and those who had no postsurgical follow-up for a period of 12 months were excluded. Among 137 patients with PD, 100 were included in the study.
Under local anesthesia, implantation of the DBS electrodes was performed bilaterally in all patients using a Leksell stereotactic frame and magnetic resonance imaging (MRI; Philips MR System Achieva, Eindhoven, the Netherlands)-guided targeting with Surgiplan (Elekta, Stockholm, Sweden). Initial values for STN localization were 12 mm lateral, 2 mm posterior, and 4 mm inferior to the mid-point between the anterior and posterior commissures. Single-track microelectrode recording (MER) using the Microdrive System (Medtronic, Inc., Minneapolis, MN, USA) was performed, and cell activity was recorded starting from 15 mm above the STN target. After the precise localization of the target point, DBS electrodes (Medtronic 3387; Minneapolis, MN, USA) with four contact points were placed in such a way that the tip of the electrode was located on the ventral boundary of the STN, passing through the center of the STN. Each contact of the DBS electrode was 1.5 mm long, and the contacts were 1.5 mm apart from each other. Based on the MER results, electrodes were positioned and labeled as follows: 0 and 1, STN; 2 and 3, the area above the STN (Fig. 1). After satisfactory outcomes during test stimulations, the position of each electrode was verified by postoperative MRI or computed tomography that was merged with the preoperatively planned target and trajectory. If the actual electrode position was acceptable, the DBS electrodes were connected to an implantable pulse generator (IPG) placed in the subclavicular area under general anesthesia. The patients underwent a single-stage operation in which both DBS electrode insertion and IPG implantation were performed on the same day. An efficacy test was performed about 1 month after surgery. Over the next 1-2 months, the contact and stimulation parameters were optimized to obtain maximum clinical benefit and minimal side effects.
The outcome assessments consisted of the Unified Parkinson's Disease Rating Scale (UPDRS) part III, UPDRS part IV, and the dyskinesia subscore of the UPDRS part IV (items 32 to 34 of UPDRS part IV) before surgery and at 12 months postoperatively. The UPDRS part III was determined for both the on-medication and off-medication states. The off-state was defined as the motor condition at 8-9 a.m. after at least 12 hours of overnight withdrawal from anti-parkinsonian medication, while the onstate was defined as the maximum improvement following a dose of levodopa equal to 150% of the patient's usual first morning dose. The UPDRS part IV and the dyskinesia subscore of the UPDRS part IV were assessed for the on-medication condition during the week prior to surgery. After implantation of the DBS device, all scores were assessed for the simulator-on condition. Additional information on the levodopa equivalent daily dose (LEDD) was obtained, both before surgery and at 12 months postoperatively. The LEDD was calculated as follows: 100 mg standard levodopa=133 mg of controlled-release levodopa=10 mg bromocriptine=1 mg pergolide=1 mg pramipexole=5 mg ropinirole.
The patients were divided into two groups based on the change in their preoperative and 12 months postoperative LEDD: Group 1, an LEDD decrease of >15%, Group 2, all other patients.
All scores from the preoperative and 12-month postoperative state were compared to assess improvement between Groups 1 and 2. The DBS electrode contact used in each Group 2 patient was also determined without reducing the LEDD. The location of each DBS contact was determined in relation to the anterodorsal boundary of the STN, which was identified by intraoperative electrophysiological mapping.
Student's t-test was used to determine whether the mean score changes differed between Groups 1 and 2. The Mann-Whitney U test was used to determine whether the mean improvement of dyskinesia differed between patients with stimulation of the area above the STN and patients with stimulation of the STN itself. All statistical analyses were performed using SPSS (version 18.0, SPSS Inc., Chicago, IL, USA). Mean values±standard deviation are presented; p values<0.05 were considered statistically significant.
This study was approved by the Institutional Review Board of our institution (IRB No. 4-2013-0182).
The patient demographics and clinical characteristics are described in Table 1. Of the 100 patients recruited, 67 were in Group 1 and 33 in Group 2. The mean ages of the patients at surgery were 55.82 and 58.70 years in Groups 1 and 2, respectively. The mean durations of disease before the operation were 11.13 years for Group 1 and 11.55 years for Group 2. There were no significant differences in patient demographics between the two groups.
At 12 months after STN DBS, the off-medication motor score (UPDRS part III) significantly decreased by 29.19% for Group 1 and 22.32% for Group 2. The on-medication motor score slightly decreased by 8.95% in Group 1, whereas in Group 2 it increased by 5.12%. There were no significant differences in the mean motor score changes between the groups in the on-medication (p=0.276) and off-medication states (p=0.123). The mean improvements of the UPDRS part IV score for Groups 1 and 2 after 12 months were 23.25% and 23.17%, respectively. No differences were observed in the mean UPDRS part IV score changes between the groups (p=0.993). The dyskinesia subscores were 4.30±2.43 at baseline and 1.87±2.52 at 12 months after surgery in Group 1, and the corresponding respective scores were 4.33±2.78 and 1.85±2.31 in Group 2. Mean dyskinesia subscore changes after 12 months were 61.90% and 57.14% for Groups 1 and 2, respectively. Group 1 was more likely than Group 2 to have an improvement of dyskinesia. However, the difference in dyskinesia improvement between the groups was not statistically significant (p=0.619) (Table 2).
Analysis of Group 2 revealed that 18 patients had an active contact above the STN, including within the zona incerta, and 15 patients had an active contact within the STN. The mean improvements of the dyskinesia subscores in patients with stimulation above the STN and within the STN after 12 months were 73.57% and 37.44%, respectively. Dyskinesia was significantly attenuated by stimulation of the area above the STN in 18 patients when compared to stimulation of the STN itself in 15 patients (p=0.048) (Fig. 2).
In this study, levodopa-induced peak-dose dyskinesia was reduced following bilateral STN DBS in all groups. This clearly shows that bilateral STN DBS can be a good therapeutic option for the treatment of dyskinesia.
Previous studies have also reported an improvement of levodopa-induced peak-dose dyskinesia following bilateral STN DBS. A randomized controlled trial by Odekerken, et al.6 demonstrated that the severity of on-phase dyskinesia, as assessed by the clinical dyskinesia rating scale (range 0-28), showed profound and significant changes from baseline (4.8) to 12 months after STN DBS (3.8). They noted that the levodopa dosage was reduced from 1254 mg/day preoperatively to 708 mg/day postoperatively. Another study by Portman, et al.2 reported that the severity of on-medication dyskinesia clearly improved by 57% 12 months after STN DBS. They noted that surgery resulted in a marked reduction of anti-parkinsonian medication (-39%) and consequently reduced the severity of peak-dose dyskinesia dramatically.
There has been some discussion regarding the mechanisms underlying the effect of STN DBS on levodopa-induced dyskinesia in patients with PD. The majority of researchers opine that the significant postoperative reduction of dyskinesia is caused by a significant postoperative reduction of levodopa medication.2719202122 In contrast to previous studies, the results of this study demonstrated that dyskinesia was improved even though the medication was unchanged, or increased, after surgery. Importantly, in PD patients with STN DBS, the improvement in dyskinesia (≈70%) was larger than the reduction in levodopa dose (35-40%) unlike for other complications. Only case reports and small series have been published about the direct effect of STN DBS on levodopa-induced dyskinesia (Table 3). Krack, et al.23 reported that high-frequency stimulation of the STN reduced the severity of peak-dose dyskinesia by 30% in response to a suprathreshold dose of levodopa. Østergaard, et al.3 described an 86% reduction in the duration of dyskinesia 12 months after bilateral STN DBS. In their study, daily levodopa dose equivalents were reduced only by 19%, which is a smaller reduction than in other similar studies. Katayama, et al.24 analyzed the direct effect of STN DBS on peak-dose dyskinesia during a 2-week period after surgery without reducing the levodopa dosage. They noted that the peak-dose dyskinesia was quickly attenuated by bipolar stimulation in 8 (18%) of the 45 patients. They also reported that dyskinesia was never attenuated by stimulation of the area above the STN. Combining our findings with those of the aforementioned studies, the improvement of levodopa-induced dyskinesia could be related directly to the effect of bilateral STN DBS.
It has been difficult to demonstrate a role for the STN in the cancellation of dyskinesia beyond the reduction in daily levodopa dose in patients. Several explanations could account for the direct antidyskinetic effect of STN DBS on levodopa-induced dyskinesia. First, most studies suggested that stimulating pallidothalamic, pallidosubthalamic, or subthalamopallidal fibers, which are densely distributed above the STN, can cause effects similar to those of thalamic or pallidal DBS.8242526 In particular, the lenticular fasciculus, which lies between the STN inferiorly and the zona incerta superiorly, is a white matter tract from the dorsal globus pallidus interna (GPi). This tract transverses the internal capsule and then combines with the ansa lenticularis (the ventral GPi outflow tracts) to form the thalamic fasciculus in the field H1 of Forel, which then terminates at the ventroanterior/ventrolateral (VA/VL) thalamic nuclei.82728 Therefore, modulation of these fibers may induce an effect similar to that of GPi DBS or Forel's field surgery. The results of this study also revealed larger improvements in dyskinesia with stimulation of the area above the STN than with stimulation of the STN itself, which is consistent with previous findings. Second, some authors have indicated that the anti-dyskinetic response after STN DBS could be attributed to the effect of continuous high-frequency electrical stimulation in the target.222329 Thus, STN surgery could induce a stable and continuous functional state with reduced fluctuations in basal ganglia activity, which mimics the effect of continuous dopamine stimulation, a process that occurs during the infusion of dopamine receptor agonists.233031 Krack, et al.23 described the effect of chronic high-frequency stimulation of the STN on peak-dose dyskinesia as being related directly to the functional inhibition of the STN and indirectly to the replacement of pulsatile dopaminergic stimulation by continuous functional inhibition of the STN. The results of their study are supported by previous observations from Nimura, et al.,32 who measured synaptic dopamine levels in the striatum using positron emission tomography with [11C]raclopride. They reported that DBS of the STN induces the stabilization of synaptic dopamine concentrations in the striatum and may contribute to the alleviation of levodopa-related motor fluctuations. A third possible mechanism is related to a dopaminergic bundle that courses through the anatomic space between the zona incerta and the STN, traveling along the lenticular fasciculus caudally and the ansa lenticularis rostrally.2733 Direct stimulation of this bundle could result in an anti-dopaminergic effect by a depolarization blockage of the axons.27
In conclusion, this study confirms the efficacy of STN DBS in ameliorating levodopa-induced dyskinesia in PD regardless of whether the levodopa dosage was reduced. Further, the improvement in levodopa-induced dyskinesia following stimulation of the area above the STN was larger than that after stimulation of the STN. Thus, in future studies, we would like to attempt simultaneous stimulation of both the STN and the area above the STN in order to diminish both the cardinal symptoms of PD and levodopa-induced dyskinesia. This combined stimulation can be performed using a single quadripolar electrode.
Although this was an unblinded retrospective study, it supports findings from previous studies investigating direct dyskinesia suppression by STN DBS. Further studies on the direct antidyskinetic effect of STN DBS in larger groups are needed to investigate the mechanism of STN DBS in patients with levodopa-induced dyskinesia.
Figures and Tables
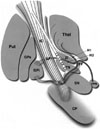 | Fig. 1Schematic illustration of the electrode insertion site as described in Hamani, et al.36 The 0 and 1 contacts were located in the STN, whereas the 2 and 3 contacts were located in the area above the STN including the zona incerta. AL, ansa lenticularis; CP, cerebral peduncle; FF, Field of Forel; GPe, globus pallidus externus; GPi, globus pallidus internus; H1, H1 Field of Forel (thalamic fasciculus); IC, internal capsule; LF, lenticular fasciculus (H2); PPN, pedunculopontine nucleus; Put, putamen; SN, substantia nigra; STN, subthalamic nucleus; Thal, thalamus; ZI, zona incerta. |
 | Fig. 2The mean improvement of dyskinesia was reduced by 73.57% (from 3.44 preoperatively to 0.83 postoperatively) in patients with stimulation of the area above the STN, whereas the mean improvement of dyskinesia was reduced by only 37.44% (from 5.40 preoperatively to 3.07 postoperatively) in patients where the STN was directly stimulated. There was a significant difference between stimulation of the area above the STN and within the STN. STN, subthalamic nucleus. |
Table 1
Clinical Characteristics of Patients

| Characteristic | Group 1 | Group 2 | p value |
|---|---|---|---|
| No. of patients | 67 | 33 | |
| Male:female | 34:33 | 11:22 | |
| Age at surgery (yrs)* | 55.82±9.08 | 58.70±8.86 | 0.964 |
| Disease duration (yrs)* | 11.13±4.66 | 11.55±5.15 | 0.496 |
Table 2
Patient Outcomes

Table 3
Comparison of Studies of Subthalamic Deep Brain Stimulation on Levodopa-Induced Dyskinesia in Patients with Parkinson's Disease

| Author, yr of publication | Patients | Improvement in dyskinesia |
|---|---|---|
| Figueiras-Méndez, et al., 199934 | 68-yr-old patient | The dyskinesia score was 15 when the stimulation was off and decreased immediately to 2 when the stimulation was switched on. The anti-parkinsonian therapy was maintained. |
| Krack, et al., 199923 | 8 patients | The severity of peak-dose dyskinesia was reduced by 30% using the same suprathreshold dose as before the operation. |
| Østergaard, et al., 20023 | 26 patients | The results showed a significant reduction of 86% in the duration of dyskinesia. Daily levodopa dose equivalents were reduced by only 19%. |
| Katayama, et al., 200624 | 45 patients | Almost complete control of the peak-dose dyskinesia was observed in 24 (53%) of the 45 patients without reducing the levodopa dosage during the early period after surgery. |
| Herzog, et al., 20078 | 3 patients | In two of three patients, additional stimulation of a proximal contact located within the subthalamic white matter may lead to a significant reduction of dyskinesia associated with STN DBS. |
| Nishikawa, et al., 201025 | 71-yr-old patient | Using contact 2 as the cathode, levodopa-induced dyskinesia was markedly attenuated. The patient received the same doses of anti-parkinsonian drugs as preoperatively. |
| Oyama, et al., 201235 | 75 patients | Despite no change in medication, 11.9% of STN DBS subjects had dyskinesia suppression. |
ACKNOWLEDGEMENTS
We would like to thank Eun Jeong Kweon (RN) for assistance with this study.
This study was financially supported by a grant from the Industrial Source Technology Development Program (no. 10033812) of the Ministry of Knowledge Economy (MKE).
References
1. Limousin P, Pollak P, Benazzouz A, Hoffmann D, Broussolle E, Perret JE, et al. Bilateral subthalamic nucleus stimulation for severe Parkinson's disease. Mov Disord. 1995; 10:672–674.

2. Portman AT, van Laar T, Staal MJ, Rutgers AW, Journee HL, Leenders KL. Chronic stimulation of the subthalamic nucleus increases daily on-time without dyskinesia in advanced Parkinson's disease. Parkinsonism Relat Disord. 2006; 12:143–148.

3. Østergaard K, Sunde N, Dupont E. Effects of bilateral stimulation of the subthalamic nucleus in patients with severe Parkinson's disease and motor fluctuations. Mov Disord. 2002; 17:693–700.

4. Simonin C, Tir M, Devos D, Kreisler A, Dujardin K, Salleron J, et al. Reduced levodopa-induced complications after 5 years of subthalamic stimulation in Parkinson's disease: a second honeymoon. J Neurol. 2009; 256:1736–1741.

5. Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006; 21:Suppl 14. S290–S304.

6. Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013; 12:37–44.

7. Russmann H, Ghika J, Combrement P, Villemure JG, Bogousslavsky J, Burkhard PR, et al. L-dopa-induced dyskinesia improvement after STN-DBS depends upon medication reduction. Neurology. 2004; 63:153–155.

8. Herzog J, Pinsker M, Wasner M, Steigerwald F, Wailke S, Deuschl G, et al. Stimulation of subthalamic fibre tracts reduces dyskinesias in STN-DBS. Mov Disord. 2007; 22:679–684.

9. Valldeoriola F, Pilleri M, Tolosa E, Molinuevo JL, Rumià J, Ferrer E. Bilateral subthalamic stimulation monotherapy in advanced Parkinson's disease: long-term follow-up of patients. Mov Disord. 2002; 17:125–132.

10. Fraix V, Pollak P, Van Blercom N, Xie J, Krack P, Koudsie A, et al. Effect of subthalamic nucleus stimulation on levodopa-induced dyskinesia in Parkinson's disease. 2000. Neurology. 2001; 57:10 Suppl 3. S60–S62.
11. Houeto JL, Damier P, Bejjani PB, Staedler C, Bonnet AM, Arnulf I, et al. Subthalamic stimulation in Parkinson disease: a multidisciplinary approach. Arch Neurol. 2000; 57:461–465.
12. Welter ML, Houeto JL, Tezenas du Montcel S, Mesnage V, Bonnet AM, Pillon B, et al. Clinical predictive factors of subthalamic stimulation in Parkinson's disease. Brain. 2002; 125(Pt 3):575–583.

13. Molinuevo JL, Valldeoriola F, Tolosa E, Rumia J, Valls-Sole J, Roldan H, et al. Levodopa withdrawal after bilateral subthalamic nucleus stimulation in advanced Parkinson disease. Arch Neurol. 2000; 57:983–988.

14. Martínez-Martín P, Valldeoriola F, Tolosa E, Pilleri M, Molinuevo JL, Rumià J, et al. Bilateral subthalamic nucleus stimulation and quality of life in advanced Parkinson's disease. Mov Disord. 2002; 17:372–377.

15. Vesper J, Klostermann F, Stockhammer F, Funk T, Brock M. Results of chronic subthalamic nucleus stimulation for Parkinson's disease: a 1-year follow-up study. Surg Neurol. 2002; 57:306–311.

16. Doshi PK, Chhaya NA, Bhatt MA. Bilateral subthalamic nucleus stimulation for Parkinson's disease. Neurol India. 2003; 51:43–48.
17. Ford B, Winfield L, Pullman SL, Frucht SJ, Du Y, Greene P, et al. Subthalamic nucleus stimulation in advanced Parkinson's disease: blinded assessments at one year follow up. J Neurol Neurosurg Psychiatry. 2004; 75:1255–1259.

18. Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003; 99:489–495.

19. Benabid AL, Benazzouz A, Limousin P, Koudsie A, Krack P, Piallat B, et al. Dyskinesias and the subthalamic nucleus. Ann Neurol. 2000; 47:4 Suppl 1. S189–S192.
20. Houeto JL, Welter ML, Bejjani PB, Tezenas du Montcel S, Bonnet AM, Mesnage V, et al. Subthalamic stimulation in Parkinson disease: intraoperative predictive factors. Arch Neurol. 2003; 60:690–694.
21. Vingerhoets FJ, Villemure JG, Temperli P, Pollo C, Pralong E, Ghika J. Subthalamic DBS replaces levodopa in Parkinson's disease: two-year follow-up. Neurology. 2002; 58:396–401.

22. Guridi J, Obeso JA, Rodriguez-Oroz MC, Lozano AA, Manrique M. L-dopa-induced dyskinesia and stereotactic surgery for Parkinson's disease. Neurosurgery. 2008; 62:311–323.

23. Krack P, Pollak P, Limousin P, Benazzouz A, Deuschl G, Benabid AL. From off-period dystonia to peak-dose chorea. The clinical spectrum of varying subthalamic nucleus activity. Brain. 1999; 122(Pt 6):1133–1146.

24. Katayama Y, Oshima H, Kano T, Kobayashi K, Fukaya C, Yamamoto T. Direct effect of subthalamic nucleus stimulation on levodopa-induced peak-dose dyskinesia in patients with Parkinson's disease. Stereotact Funct Neurosurg. 2006; 84:176–179.

25. Nishikawa Y, Kobayashi K, Oshima H, Fukaya C, Yamamoto T, Katayama Y, et al. Direct relief of levodopa-induced dyskinesia by stimulation in the area above the subthalamic nucleus in a patient with Parkinson's disease--case report. Neurol Med Chir (Tokyo). 2010; 50:257–259.

26. Alterman RL, Shils JL, Gudesblatt M, Tagliati M. Immediate and sustained relief of levodopa-induced dyskinesias after dorsal relocation of a deep brain stimulation lead. Case report. Neurosurg Focus. 2004; 17:E6.
27. Brodsky MA, Hogarth P, Nutt JG. OFF-off rebound dyskinesia in subthalamic nucleus deep brain stimulation of Parkinson's disease. Mov Disord. 2006; 21:1487–1490.

28. Peppe A, Pierantozzi M, Bassi A, Altibrandi MG, Brusa L, Stefani A, et al. Stimulation of the subthalamic nucleus compared with the globus pallidus internus in patients with Parkinson disease. J Neurosurg. 2004; 101:195–200.

29. Rodriguez-Oroz MC, Zamarbide I, Guridi J, Palmero MR, Obeso JA. Efficacy of deep brain stimulation of the subthalamic nucleus in Parkinson's disease 4 years after surgery: double blind and open label evaluation. J Neurol Neurosurg Psychiatry. 2004; 75:1382–1385.

30. Obeso JA, Rodriguez-Oroz MC, Rodriguez M, DeLong MR, Olanow CW. Pathophysiology of levodopa-induced dyskinesias in Parkinson's disease: problems with the current model. Ann Neurol. 2000; 47:4 Suppl 1. S22–S32.
31. Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005; 128(Pt 10):2240–2249.

32. Nimura T, Yamaguchi K, Ando T, Shibuya S, Oikawa T, Nakagawa A, et al. Attenuation of fluctuating striatal synaptic dopamine levels in patients with Parkinson disease in response to subthalamic nucleus stimulation: a positron emission tomography study. J Neurosurg. 2005; 103:968–973.
33. Prensa L, Cossette M, Parent A. Dopaminergic innervation of human basal ganglia. J Chem Neuroanat. 2000; 20:207–213.
34. Figueiras-Méndez R, Marín-Zarza F, Antonio Molina J, Jiménez-Jiménez FJ, Ortí-Pareja M, Magariños C, et al. Subthalamic nucleus stimulation improves directly levodopa induced dyskinesias in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999; 66:549–550.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download