Abstract
Purpose
The TWIK-related spinal cord K+ channel (TRESK) has recently been discovered and plays an important role in nociceptor excitability in the pain pathway. Because there have been no reports on the TRESK expression or its function in the dorsal horn of the spinal cord in neuropathic pain, we analyzed TRESK expression in the spinal dorsal horn in a spinal nerve ligation (SNL) model.
Materials and Methods
We established a SNL mouse model by using the L5-6 spinal nerves ligation. We used real-time polymerase chain reaction and immunohistochemistry to investigate TRESK expression in the dorsal horn and L5 dorsal rot ganglion (DRG).
Results
The SNL group showed significantly higher expression of TRESK in the ipsilateral dorsal horn under pain, but low expression in L5 DRG. Double immunofluorescence staining revealed that immunoreactivity of TRESK was mostly restricted in neuronal cells, and that synapse markers GAD67 and VGlut2 appeared to be associated with TRESK expression. We were unable to find a significant association between TRESK and calcineurin by double immunofluorescence.
Neuropathic pain is well known as a leading cause of chronic pain, but there is still no solid treatment. Even the strongest analgesics, such as narcotics, are not effective against excruciating nature of neuropathic pain. The exact cause, pathophysiology and mechanism remain unclear.
The molecular structure, electrophysiological and pharmacological properties, and function of the two-pore domain K+ channel (K2p) have recently been described. K2p channel is now known to be deeply involved in cellular excitability. Leaking of potassium currents from K2p contributes to resting membrane potential, and regulates cell firing.12
The K2p channel family is divided into 6 subfamilies by channel structure and regulatory mechanism. The TWIK-related spinal cord K+ channel (TRESK) was most recently discovered and has received particular attention for its role in nociception.3 TRESK is strongly expressed in the dorsal root ganglion (DRG), and low-level TRESK expression after axotomy is closely involved in the transmission of nociception and the onset and development of neuropathic pain.4 Studies on peripheral nerve injury and several pain models have demonstrated that hyperexcitability and ectopic discharge of the DRG can affect pain perception leading to spontaneous pain, hyperalgesia, and allodynia.567 Hyperexcitability in the DRG or spinal dorsal horn is the basic mechanism of neuropathic pain in several pain models.578 If K2p acts as a major regulator of excitability in the spinal cord, it is highly likely that it is also involved in the mechanism underlying neuropathic pain.
There have been no reports on the TRESK expression or its function in the dorsal horn of the spinal cord in neuropathic pain. Therefore, we analyzed the TRESK expression in the spinal dorsal horn after sustained mechanical allodynia in a spinal nerve ligation (SNL) model.9
Male Sprague-Dawley rats weighing 180-200 g were divided into plastic containers containing clean sawdust (≤3 rats per container), and were provided food and water ad libitum. The breeding room was naturally controlled with light every 12 hours and maintained at consistent temperature (-23℃) and humidity (-50%). All experiments were approved by the Chungnam Medical School's Animal Experiment Committee.
Animals were divided into sham (n=8) and SNL (n=8) groups. SNL was performed on the left side under gaseous anesthesia with a mixture of isoflurane (0.8 vol %) and a 1:1 flow ratio of N2O and O2. No surgical procedure was performed on the right side. Rats were placed in a prone position, and the left paraspinal muscles were separated from the spinous processes at L4-S2. The L5 transverse process was carefully removed with a small rongeur to visually identify the L5-6 spinal nerves. The left L5 nerve was isolated and tightly ligated with 3-0 silk thread. Complete hemostasis was confirmed and the wound was sutured. The same procedures, except SNL, were performed in the sham group.
Mechanical paw withdrawal thresholds (PWTs) were measured with the up-down testing paradigm on days 0, 3, 7, 10, and 14 after SNL or sham surgery.10 Von Frey filaments (39337500, Touch Test™ Sensory Evaluator Kit of 20) in log increments of force (0.007, 0.016, 0.4, 0.6, 1, 1.4, 2, 4, 6, 8, 10, 15 g) were applied for 4-6 s to the region between foot pads in the plantar aspect of the hind paw. The 2-g stimulus was applied first. If a positive response occurred, the next smaller von Frey filament was used; if a negative response was observed, the next higher force was used.
Immunohistochemistry was performed 14 days after surgery. Rats were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally) and perfused transcardially with heparinized phosphate-buffered saline (PBS, pH 7.4), followed by perfusion with 4% paraformaldehyde for 15 min. Lumbar enlargement (L4-6) regions of the spinal cords and lumbar 5th DRG were immediately removed, immersed in the same fixative overnight, and embedded in paraffin.
Four-micron sections of the paraffin-embedded tissue arrays were deparaffinized, rehydrated in graded alcohol, and microwaved for 15 min in citrate buffer (pH 6.0). Antigen was retrieved with 0.01 M citrate buffer (pH 6.0) by heating the sample in a microwave vacuum histoprocessor (RHS-1, Milestone, Bergamo, Italy) at a controlled final temperature of 121℃ for 15 min. For immunohistochemical analyses, endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide. The sections were treated with Protein Block solution (Dako, Glostrup, Denmark) for 20 min and then incubated with specific polyclonal antisera against TRESK [1:1000 rabbit polyclonal KCNK18/TRESK antibody (catalog number: ab83930, Abcam, USA)] overnight in a humid chamber at 4℃. After washing with PBS, the tissues were exposed to biotinylated anti-rabbit IgG and streptavidin peroxidase complex (Vector Laboratories, USA). Immunostaining was visualized with diaminobenzidine and the specimens were mounted using Polymount (Polysciences, USA). Our preliminary results showed negative data in the antibody absorption test of TRESK antibody.
In order to simultaneously observe a pair of antigens in the same section, rabbit TRESK was used with one of the following monoclonal antibodies: anti-glial fibrillary acidic protein (GFAP, 1:1000, #AM020, Biogenex), anti-NeuN (1:100, MAB377, Millipore), calcineurin α (1:500, #2614, Cell Signaling), anti-VGlut2 (anti-vesicular glutamate transporter 2, 1:1000, ab79157, Abcam), or anti-GAD67 (anti-glutamic acid decarboxylase 67, 1:1000, #MAB5406, Millipore). Sections were immunoreacted with TRESK as described above, but a Cy3-conjugated anti-rabbit secondary antibody was used (1:500, Amersham, UK). Sections were further processed for GFAP and NeuN immunocytochemistry and Cy2-conjugated anti-mouse secondary antibody (1:200, Amersham, UK) counterstained with 40,6-diamidino-2-phenylindole. Double-stained sections were analyzed with an Axiophot microscope (CarlZeiss, Germany).
Tissue sections were examined by dark-field microscopy (Zeiss Axioscope, Germany), and gray matter landmarks were identified based on previous reports to determine the segmental level and define individual spinal cord laminae.11 For quantitative analysis of TRESK-immunoreactive (IR) cells, 5 spinal cord sections from the lumbar-enlarged region were randomly selected from each animal and scanned. Individual sections were digitized with 4096 gray levels using an Axio Scope A1 (Carl Zeiss, Germany) and a computer-assisted image analysis system (Image J, NIH, USA). In order to maintain a constant threshold for each image and to compensate for subtle variability in immunostaining, we counted only neurons that were at least 85% darker than the average gray level in each image after background subtraction and shading correction. Microscope illumination and data acquisition settings were fixed throughout the analysis procedure. The average number of TRESK IR cells per section from each animal was obtained, and values from at least 5 rats in each group were averaged and presented as group data. TRESK IR expression was quantified in superficial dorsal horn (SDH) laminae I and II. These regions were identified based on cytoarchitectonic criteria as defined previously.11 All numerical results related to TRESK IR profiles in each dorsal horn region are expressed as the number of TRESK IR-positive cells per unit area. All quantitation procedures were performed blindly with regard to the experimental condition of each animal.
Total RNA was isolated from the spinal cord with Trizol Reagent (Ambion, USA). cDNA was synthesized using 1 µg of total RNA and the QuantiTect Reverse Transcription Kit (Qiagen, CA, USA). Amounts of total RNA were analyzed by Power SYBR Green PCR Master Mix (Applied Biosystems, CA, USA), Rn_KCNK18_1_SG (QT00447048), Rn_Ppp3ca_2_SG (QT00495026), and Rn_Gapd_1_SG (QT00199633), QuantiTect primer Assays (Qiagen, USA), and a StepOne Plus system (Applied Biosystems, USA). PCR conditions were as follows: 10 min at 95℃, and 40 cycles of 15 s at 95℃ followed by 1 min at 60℃. Relative fold changes in KCNK18 gene expression were calculated by the ΔΔCT method using GAPDH as the normalization reference.12
Results from the behavioral study, qPCR, and immunohistochemistry were statistically analyzed by one-way or two-way analysis of variance (ANOVA). Data are presented as means±SEM. When ANOVA showed a significant difference, pair-wise comparisons between means were tested by the Newmann-Keuls Comparison Test. Significance was set at p<0.05. The statistical software package SigmaStat (Systat, USA) was used for all statistical analysis.
Neuropathic pain in the form of allodynia developed in the SNL group, while there was no such pain in the sham group (Fig. 1). Mechanical allodynia occurred after SNL. PWTs in response to von Frey filament stimulation decreased from 6.67±0.94 g before surgery to 0.57±0.07 g 10 days after surgery. This mechanical allodynia persisted for at least 14 days (0.65±0.13 g). Sham-operated rats showed no signs of hypersensitivity.
TRESK was expressed in the dorsal horn of the spinal cord. The bilateral region of the sham group and the contralateral region of the SNL group showed similar TRESK expression (Fig. 2-1). However, the SNL group showed significantly higher expression of TRESK in the ipsilateral region under pain in contrast to the contralateral region (Fig. 2-2). Increased TRESK expression was most prominent in the SDH (laminae I-II, p<0.001) (Fig. 2-2D). In short, TRESK expression significantly increased in the ipsilateral region where mechanical allodynia due to SNL occurred, and this was most prominent in the SDH.
TRESK immunoreactivity decreased in the DRG of SNL rats (Fig. 3). Fourteen days following SNL, TRESK dramatically decreased in the ipsilateral DRG in comparison to the contralateral side.
TRESK is expressed exclusively in spinal cord neurons 14 days after SNL in rats. Double immunofluorescence staining in the ipsilateral lumbar spinal dorsal horn between TRESK, neuron, and astrocyte markers showed that TRESK was mainly located in neurons, but not astrocytes at 14 days (Fig. 4).
TRESK gene expression increased in the ipsilateral lumbar spinal cord at 0, 7, 14, 21, and 28 days after SNL in rats. TRESK transcript expression increased in the dorsal horn of SNL rats (p<0.05) (Fig. 5).
To identify the relationship between inhibitory and excitatory synapses, double immunofluorescence of TRESK-expressing neurons was performed 14 days after SNL in the ipsilateral spinal dorsal horn with synapse markers GAD67 and VGlut2. As shown in Fig. 6-1 and -2, both appeared to be associated with TRESK expression.
Czirják and Enyedi13 reported that calcineurin may play a major role in TRESK activation. However, we were unable to find a significant association between TRESK and calcineurin by double immunofluorescence (Fig. 7). qPCR performed 0, 7, 14, 21, and 28 days after SNL in the ipsilateral dorsal horn also showed no significant difference, although there was an increased tendency at 21 days (Fig. 8).
Central or peripheral nerve injuries can cause consistent hyperactivity in the sensory neuron, leading to ectopic discharges that eventually cause spontaneous, neuropathic pain.11 Ion channels such as voltage-dependent Na+, K+, Ca2+ channels are deeply involved in hyperexcitability after neural injury.141516 The recently reported K2P channel is widely expressed in the central nervous system and regulates central neuronal excitability.3 TRESK currents are increased up to 3-fold by clinical concentrations of isoflurane, halothane, sevoflurane, and desflurane.17 TRESK also plays an important role in nociceptor excitability18 and in the pain pathway, therefore, TRESK is likely to be a major target of many volatile anesthetics and neuroprotective agents.
Kang and Kim19 reported that the background K+ current of K2P channels in the DRG regulates DRG cell excitability; channel inhibition causes increased excitability. Tulleuda, et al.4 reported that axotomy inhibits TRESK expression in the DRG, which may contribute to increased excitability after nerve injury. In normally behaving animals, pharmacological inhibition of TRESK channel activity increases pain sensitivity and painful animal behavior, supporting an important role for TRESK in nociceptor excitability.20
Immunostaining also showed changes in TRESK expression after sustained mechanical allodynia due to SNL. In other words, TRESK is indeed down-regulated in the DRG as shown in previous report,4 but it is also upregulated-most prominently in the SDH. Our results showed increased expression of TRESK in the dorsal horn after onset of neuropathic pain. This is the first study to show increased TRESK expression in a pain model, although previous studies using a different pain model showed decreased TRESK expression in the DRG.421 These contradictory results could be due to pain transmission in neuropathic pain, as TRESK may have different roles in individual pain modulation pathways. Another possibility is channel dysfunction in the SDH due to increased excitability in neuropathic pain.
Wu, et al.22 reported that hypoxic damage induces the activity of TREK-1, a K2P channel related to glutamate clearance capacity in astrocytes. Zhou, et al.23 reported that intrathecal injection of TRESK gene recombinant adenovirus in spared nerve injured rats upregulated TRESK expression in the DRG, and that neuropathic pain was decreased by attenuating astrocyte activity. This could be due to decreased neuronal excitability in the pain pathway caused by increased TRESK expression.
Two previous studies have reported that in the state of neuronal excitability, increased K2P may prevent neuronal cell death and reduce neuropathic pain.22 Berliocchi, et al.24 suggested compensation in the case of nerve death in the spinal cord posterior horn after peripheral nerve injury. The SDH acts as a gate for pain transmission, and the gate control theory by Melzack and Wall25 is useful in understanding the pain control mechanism. Increased TRESK expression in the ipsilateral SDH may be considered a compensatory mechanism in neuropathic pain, in order to control increased neural excitability. This may explain the increased TRESK expression which peaks 21 days after SNL.
Several previous studies about neuropathic pain models have reported cell death in the CNS.26272829 Leong, et al.30 reported a 23% decrease in rostral ventromedial medulla neurons in the side ipsilateral to SNL. It is possible that the DRG, the primary neuron in the dorsal horn, is activated after spinal nerve injury, leading to dorsal horn cell activation, increased excitatory peptide, and central sensitization of secondary neurons. Due to increased excitability of the spinal dorsal horn after peripheral nerve injury, dysfunction or cell death of secondary spinal neurons may occur. This may explain our finding of secondary neurons in the ipsilateral SDH, leading to TRESK channel dysfunction and increased expression in the neuron cell membrane.
We also performed double immunohistochemistry to determine the role of TRESK-expressing neurons in the ipsilateral spinal dorsal horn with excitatory and inhibitory synapse markers. Our results indicated that GAD67 and VGlut2 are involved in TRESK-expressing neurons.
Czirják, et al.1331 suggested that calcineurin could play an important role in TRESK activation or pain transmission. In our study, TRESK expression was not regulated exclusively by calcineurin in the ipsilateral SDH in 5th , 6th lumbar SNL. Calcineurin also increased 21 days after SNL in the spinal dorsal horn, but the change was not statistically significant. This could be due to different pain models used in each study and additional studies may be needed to understand the role of calcineurin and TRESK.
In conclusion, We have demonstrated that SNL upregulates TRESK expression in the dorsal horn and increases pain sensitivity and painful animal behavior. To our best knowledge, this is the first report of increased TRESK expression in a pain model. Unlike the involvement of DRG TRESK expression, however, we suspect that the increased TRESK expression of the SDH in our pain model may have a different role in neuropathic pain. Therefore, more studies are needed to understand the relationship between neuropathic pain and TRESK upregulation in the SDH after SNL.
Figures and Tables
Fig. 1
Mechanical allodynia after spinal nerve ligation (SNL). Paw withdrawal threshold in response to von Frey filament stimulation decreased from 6.67±0.94 g before surgery to 0.57±0.07 g 10 days after surgery. This mechanical allodynia persisted at least for 14 days (0.65±0.13 g). Sham-operated rats showed no signs of hypersensitivity. *p<0.001 vs. the corresponding contralateral side. co, contralateral to the injured hind paw; ips, ipsilateral to the injured hind paw.

Fig. 2-1
TRESK immunoreactivity was similar in the spinal dorsal horn of the sham group. The bilateral region of the sham group showed similar TRESK expression. Scale bars=100 µm in A; 50 µm in B and C. TRESK, TWIK-related spinal cord K+.

Fig. 2-2
TRESK immunoreactivity (IR) increased in the spinal dorsal horn of spinal nerve ligation (SNL) rats. Fourteen days following SNL, TRESK IR dramatically increased in the ipsilateral dorsal horn (C) vs. the contralateral side (B). Quantitative measurement of TRESK IR cells in the L5 spinal dorsal horn (D). The mean number of TRESK IR cells in a fixed area occupied in ipsilateral superficial laminae of the dorsal horn (dot-line) was significantly higher than their contralateral counterparts (*p<0.05) (mean±SEM, n=8). Scale bars=100 µm in A; 50 µm in B and C. TRESK, TWIK-related spinal cord K+.
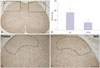
Fig. 3
TRESK immunoreactivity was similar in the both DRG of sham group (A and B). 14 days after spinal nerve ligation (SNL), TRESK expression was dramatically reduced in the ipsilateral DRG (D) vs. the contralateral side (C). Scale bar=20 µm. TRESK, TWIK-related spinal cord K+; DRG, dorsal root ganglion.
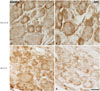
Fig. 4
TRESK is expressed exclusively in neurons in the spinal dorsal horn 14 days after spinal nerve ligation (SNL). Double immunofluorescence staining in the ipsilateral lumbar spinal dorsal horn of TRESK (red; A and E) and NeuN, a neuronal marker (green; B) or GFAP, an astrocyte marker (green; F); TRESK was mainly located in neurons, but not astrocytes 14 days after SNL (C, D, G, H). Scale bar=20 µm. TRESK, TWIK-related spinal cord K+; GFAP, glial fibrillary acidic protein; DAPI, 40,6-diamidino-2-phenylindole.
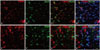
Fig. 5
TRESK transcript expression increased in the dorsal horn of spinal cord of SNL rats. Quantitative real-time PCR of TRESK gene expression in the ipsilateral L5-6 spinal cord at 0, 7, 14, 21, and 28 days after SNL. Data are presented as the fold change from the control (naïve) mean±SEM, which represented normalized averages derived from the threshold cycles in 6 to 8 ipsilateral samples. *p<0.05 vs. control, †p<0.01 vs. control. TRESK, TWIK-related spinal cord K+; SNL, spinal nerve ligation; PCR, polymerase chain reaction.

Fig. 6-1
Immunofluorescence staining of TRESK (red) and GAD67 (green) in the spinal dorsal horn of SNL rats. TRESK expression is associated with inhibitory synapses. Scale bars=50 µm in A, B, and C; 20 µm in D, E, and F. TRESK, TWIK-related spinal cord K+; SNL, spinal nerve ligation.
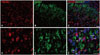
Fig. 6-2
Immunofluorescence staining of TRESK (red) and VGlut2 (green) in the spinal dorsal horn of SNL rats. TRESK expression is associated with excitatory synapses. Scale bars=50 µm in A, B, and C, 20 µm in D, E, and F. TRESK, TWIK-related spinal cord K+; SNL, spinal nerve ligation.
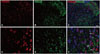
Fig. 7
Immunofluorescence staining of TRESK (red) and calcineurin α (green) in the spinal dorsal horn of SNL rats. TRESK expression was not regulated exclusively by calcineurin in the ipsilateral L5-6 region of SNL rats. Scale bars=50 µm in A-D; 20 µm in E-H. TRESK, TWIK-related spinal cord K+; SNL, spinal nerve ligation.
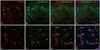
Fig. 8
qPCR of calcineurin gene expression in the L5-6 ipsilateral lumbar enlargement of spinal cord at 0, 7, 14, 21, and 28 days after spinal nerve ligation. Data are presented as fold change from control mean±SEM, which represented normalized averages derived from the threshold cycles in 6 to 8 ipsilateral samples.

References
1. Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005; 11:2717–2736.

2. Ocaña M, Cendán CM, Cobos EJ, Entrena JM, Baeyens JM. Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol. 2004; 500:203–219.

3. Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010; 90:559–605.

4. Tulleuda A, Cokic B, Callejo G, Saiani B, Serra J, Gasull X. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol Pain. 2011; 7:30.

5. Chung JM, Chung K. Importance of hyperexcitability of DRG neurons in neuropathic pain. Pain Pract. 2002; 2:87–97.

6. Song XJ, Vizcarra C, Xu DS, Rupert RL, Wong ZN. Hyperalgesia and neural excitability following injuries to central and peripheral branches of axons and somata of dorsal root ganglion neurons. J Neurophysiol. 2003; 89:2185–2193.

7. Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009; 89:707–758.

9. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992; 50:355–363.

10. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63.

11. Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol. 2001; 85:630–643.

12. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.

13. Czirják G, Enyedi P. Targeting of calcineurin to an NFAT-like docking site is required for the calcium-dependent activation of the background K+ channel, TRESK. J Biol Chem. 2006; 281:14677–14682.

14. Abdulla FA, Smith PA. Changes in Na(+) channel currents of rat dorsal root ganglion neurons following axotomy and axotomy-induced autotomy. J Neurophysiol. 2002; 88:2518–2529.

15. Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol. 2001; 85:644–658.

17. Liu C, Au JD, Zou HL, Cotten JF, Yost CS. Potent activation of the human tandem pore domain K channel TRESK with clinical concentrations of volatile anesthetics. Anesth Analg. 2004; 99:1715–1722.

18. Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, et al. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem. 2003; 278:27406–27412.

19. Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006; 291:C138–C146.
21. Marsh B, Acosta C, Djouhri L, Lawson SN. Leak K+ channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol Cell Neurosci. 2012; 49:375–386.

22. Wu X, Liu Y, Chen X, Sun Q, Tang R, Wang W, et al. Involvement of TREK-1 activity in astrocyte function and neuroprotection under simulated ischemia conditions. J Mol Neurosci. 2013; 49:499–506.

23. Zhou J, Yang CX, Zhong JY, Wang HB. Intrathecal TRESK gene recombinant adenovirus attenuates spared nerve injury-induced neuropathic pain in rats. Neuroreport. 2013; 24:131–136.

24. Berliocchi L, Russo R, Tassorelli C, Morrone LA, Bagetta G, Corasaniti MT. Death in pain: peripheral nerve injury and spinal neurodegenerative mechanisms. Curr Opin Pharmacol. 2012; 12:49–54.

26. Fuccio C, Luongo C, Capodanno P, Giordano C, Scafuro MA, Siniscalco D, et al. A single subcutaneous injection of ozone prevents allodynia and decreases the over-expression of pro-inflammatory caspases in the orbito-frontal cortex of neuropathic mice. Eur J Pharmacol. 2009; 603:42–49.

27. Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005; 25:7317–7323.

28. Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990; 42:205–213.

29. Whiteside GT, Munglani R. Cell death in the superficial dorsal horn in a model of neuropathic pain. J Neurosci Res. 2001; 64:168–173.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download