Abstract
Purpose
Dickkopf-1 (DKK-1) is a Wnt/β-catenin signaling pathway inhibitor. We investigated whether DKK-1 is related to progression in hepatocellular carcinoma (HCC) cells and HCC patients.
Materials and Methods
In vitro reverse-transcription polymerase chain reaction (RT-PCR), wound healing assays, invasion assays, and ELISAs of patient serum samples were employed. The diagnostic accuracy of the serum DKK-1 ELISA was assessed using receiver operating characteristic (ROC) curves and area under ROC (AUC) analyses.
Results
RT-PCR showed high DKK-1 expression in Hep3B and low in 293 cells. Similarly, the secreted DKK-1 concentration in the culture media was high in Hep3B and low in 293 cells. Wound healing and invasion assays using 293, Huh7, and Hep3B cells showed that DKK-1 overexpression promoted cell migration and invasion, whereas DKK-1 knock-down inhibited them. When serum DKK-1 levels were assessed in 370 participants (217 with HCC and 153 without), it was significantly higher in HCC patients than in control groups (median 1.48 ng/mL vs. 0.90 ng/mL, p<0.001). The optimum DKK-1 cutoff level was 1.01 ng/mL (AUC=0.829; sensitivity 90.7%; specificity 62.0%). Although DKK-1 had a higher AUC than alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) (AUC=0.829 vs. 0.794 and 0.815, respectively), they were statistically similar (all p>0.05). When three biomarkers were combined (DKK-1 plus AFP plus DCP), they showed significantly higher AUC (AUC=0.952) than single marker, DKK-1 plus AFP, or DKK-1 plus DCP (all p<0.001).
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and constitutes over 5% of all cancers.12 Its worldwide incidence is estimated at 500000 to 1 million new cases per year.3 The development of HCC has a multi-step carcinogenetic process similar to other solid tumors; however, it possesses unique risk factors. Hepatitis B (HBV) or C (HCV) viral infection, heavy alcohol intake, prolonged dietary exposure to aflatoxin or vinyl chloride, and primary hemochromatosis are all associated with HCC development.4 Patients with HCC usually have at least one of these risk factors and their prevalence varies between patients according to their geographical origin.5 This HCC manifestation diversity is also found at the genetic and epigenetic level, and a large number of these alterations have been described in the literature.6 Of these, alterations in Wnt/β-catenin signaling have been proved to play a critical role in HCC development.7 Therefore, comprehensive knowledge of the Wnt/β-catenin signaling pathway is essential to identify potential endogenous molecular targets of novel drugs.
Secreted Wnt antagonists play important roles in regulating Wnt/β-catenin signaling.8910 Of these, dickkopf-1 (DKK-1), a prototypical member of a secreted protein family, has been known as a potent antagonist of Wnt/β-catenin signaling.1011 DKK-1 acts as an inhibitory ligand of the low-density lipoprotein receptor-related protein 5/6 co-receptors and subsequently blocks their interaction with Wnt, resulting in β-catenin degradation.121314 It has been reported that DKK-1 is down-regulated in human colon tumors, indicating that DKK-1 may act as a tumor suppressor and that high DKK-1 expression indicated favorable responses to chemotherapy in brain tumors.1516
Conversely, DKK-1 overexpression was found in human hepatoblastomas, Wilms' tumors, and multiple myelomas.1718 In addition, Forget, et al. reported high DKK-1 expression in 21 out of 73 cases of breast cancer, particularly in hormone-resistant breast tumors, and several experimental studies have shown that DKK-1 was highly expressed in HCC.2021 Another study showed that DKK-1 expression was significantly higher in specimens with poorer pathologic grade and higher 18F-fluorodeoxyglucose positron emission tomography uptake in HCC.22 Furthermore, a recent study reported that elevated DKK-1 expression is a critical event in HCC patients, indicating poor clinical outcomes, and that DKK-1 is a novel prognostic predictor in these patients.23 All these studies suggest that DKK-1 is associated with tumor growth and poor prognosis in some malignant tumors.
The controversies regarding the molecular and clinical implications of DKK-1 molecules remain unresolved. In this study, therefore, we investigated the role of DKK-1 in the Wnt/β-catenin signaling pathway using several HCC cell lines and compared the diagnostic accuracy of serum DKK-1 to the conventional HCC tumor markers, alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) in human patients.
Hep3B and 293 cells were grown in modified Eagle's medium (MEM, GIBCO BRL, Grand Island, NY, USA). Huh7 was grown in Dulbecco's MEM (DMEM, GIBCO BRL) supplemented with 10% fetal bovine serum (GIBCO BRL), 2 mM glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin. All cells were cultured at 37℃ in a 5% CO2 atmosphere.
All serum samples were collected from Yonsei Liver Blood Bank (YLBB) system (approval number, 4-2009-0725), Severance Hospital, Sinchon-dong, Korea. This study was approved by the independent Institutional Review Board (IRB) of Severance Hospital and conformed to the 1975 Helsinki declaration ethical guidelines.
Our exclusion criteria were as follows: 1) previous history of anti-HCC treatment (n=25), 2) pathological or radiological evidence of mixed HCC-cholangiocellular carcinoma (n=5), 3) insufficient clinical data (n=2), and 4) insufficient serum samples (n=10). Serum samples from 80 HCC patients who underwent surgical resection, 137 HCC patients who received non-surgical anti-HCC treatments (chemotherapy, radiotherapy, ablation, and conservative care), 67 patients with liver cirrhosis alone, 46 patients with chronic hepatitis alone, and 40 healthy subjects with no evidence of viral hepatitis [HBV surface antigen (HBsAg) and HCV antibody negative and normal liver biochemistry] were collected between February 2010 and April 2011.
HCC diagnosis was made on the following criteria: 1) pathological HCC diagnosis for patients who underwent surgical resection or percutaneous biopsy or 2) clinical and radiological HCC diagnosis for patients without available HCC tissue specimens, based on the guidelines of the American Association for the Study of Liver Diseases.24 In the surgical resection cases, Tumor-Node-Metastasis (TNM) staging based on the American Joint Commission on Cancer (7th edition) was assessed postoperatively.25 Otherwise, HCC stage was clinically defined according to the Barcelona Clinic Liver Cancer (BCLC) staging system.26 For this study, early-stage HCC was defined as HCCs with TNM I-II and BCLC A-B.
Liver cirrhosis was histologically or clinically diagnosed as follows: 1) platelet count <100000/µL and ultrasonographic findings suggestive of cirrhosis, including a blunted, nodular liver edge accompanied by splenomegaly (>12 cm), 2) esophageal or gastric varices, or 3) overt liver cirrhosis complications, including ascites, variceal bleeding, and hepatic encephalopathy.2728 Liver function was graded according to the Child-Pugh classification.
During histological examinations, tumor differentiation was classified according to the Edmondson grading system. Microvascular invasion was defined as a tumor within the vascular space of a capillary in the tumor capsule or the sinusoidal space of a non-tumoral liver that was visible only upon microscopy. Portal vein invasion was defined as a tumor within the vascular space of a portal vein in the portal tract of a non-tumoral liver that was visible only upon microscopy.29 Satellite nodules were defined as HCC nodules that existed in the same segment of the main tumor, but remained separated by an interval of non-tumoral liver parenchyma.30 Background liver histology was evaluated semiquantitatively according to the Batts and Ludwig31 scoring system. Fibrosis was staged on a 0 to 4 scale: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with a few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis. Activity was graded as A0, none; A1, minimal; A2, mild; A3, moderate; and A4, severe activity.
Total RNA was isolated using an RNeasy kit (Qiagen, Santa Clarita, CA, USA). cDNA was synthesized by reverse transcription with 1µg of total RNA, 0.5 µg of random hexamers (Promega, Madison, WI, USA), 1.25 mM dNTP (Boehringer-Mannheim, Mannheim, Germany), and 200 U Moloney murine leukemia virus reverse transcriptase (MMLV-RT, Invitrogen, Carlsbad, CA, USA) in a 20 µL reaction volume. Polymerase chain reaction (PCR) was performed with 3 µL of cDNA, 10 pmol of the primer sets, 0.25 mM dNTP, and 2 U of Taq polymerase (Perkin-Elmer, Norwalk, CT, USA). PCR cycling conditions were as follows: 23-35 cycles of denaturation at 94℃ for 30 s, annealing at 56-60℃ for 30 s, and extension at 72℃ for 30 s. The primer sequences used were as follows: DKK-1-forward, 5'-TCACACCAAAGGACAAGAAG-3'; DKK-1-reverse, 5'-ATCTTGGACCAGAAGTGTCTAGC-3'; β-catenin-forward, 5'-ACAAACTGTTTTGAAAATCCA-3'; β-catenin-reverse, 5'-CGAGTCATTGCATACTGTCC-3'; GAPDH-forward, 5'-GCACTCAGGCTGAGAAC-3'; and GAPDH-reverse, 5'-GGTGAAGACGCCAGTGGA-3'. PCR products were analyzed by agarose gel electrophoresis.
To evaluate TCF-4/β-catenin transcriptional activity, the luciferase reporter assay was performed using the luciferase reporter constructs, TOPflash and FOPflash. TOPflash contains three copies of the TCF-4 binding site and FOPflash contains mutated TCF-4 binding sites. Hep3B and 293 cells were cultured to 70% confluence in 24-well plates. Then, TOPflash and FOPflash plasmids were transfected using Fugene HD Reagent (Promega) according to the manufacturer's instructions. Following transfection, the luciferase activity was determined using the dual-luciferase reporter assay system (Promega). All luciferase activities were normalized to renilla luciferase activity (internal control) using a luminometer (Centro XS3 LB960, Berthold.Tec, Bad Wildbad, Germany). Luciferase assay data from three independent experiments were analyzed.
For DKK-1 overexpression, 293 and Huh7 cells were transfected with either pcDNA3.1-Luciferase (control) or the same vector containing the DKK-1 coding sequence. Briefly, cells were seeded into plates and grown for 24 h until they reached 50-60% confluence, followed by transfection with the DKK-1 or control vector using the Fugene HD transfection reagent. DKK-1 overexpression was confirmed using ELISA (R&D Systems, Minneapolis, MN, USA). Small interfering RNA (siRNA) were transfected when the cells reached 70% confluence. The DKK-1 siRNAs (siDKK-1, NM012242) and GFP siRNA (siGFP, SP-2011) were purchased from Bioneer (Daejeon, Korea). Experiments were conducted using Fugene HD transfection reagent and 100 nM siRNA. The DKK-1 knock-down was confirmed using ELISA and measured at 450 nm using a spectrophotometric microplate reader (Molecular Devices, Toronto, Canada).
For the wound healing assay, Huh7 or 293 cells were seeded into 35 mm dishes (Corning, NY, USA). Once the cells reached 100% confluence, wound healing assays were performed by scratching a sterile pipette tip through the confluent monolayer. The cells were then washed, and cultured for another 24 h before microscopic observation (Olympus America, Melville, NY, USA) at 40× magnification. The wound healing assay was performed in triplicate.
For the trans-well invasion assay, 1×105 Huh7 cells in serum-free medium were placed into the upper chamber of the trans-well insert (Corning Costar, Cambridge, MA, USA) with Matrigel (BD Biosciences, San Jose, MA, USA). After 24 h of incubation at 37℃, the cells in the upper chamber and membrane were removed, the remaining cells stained with hematoxylin (Dako, Carpinteria, CA, USA) and eosin (Sigma, ST. Louis, MO, USA), and the number of cells adhering to the lower membrane of the inserts was counted. For each experimental group, the invasion assay was performed in triplicate. Three randomly selected fields in each replicate were chosen for cell number quantification.
Cell growth in Huh7 cells was analyzed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, cells were seeded into a 96-well plate, left overnight to adhere, and treated with siDKK-1. Following treatment, 50 µL of MTT solution (2 mg/mL in PBS) was added to each well and incubated for 4 h at 37℃. The supernatant was discarded and 150 µL of dimethyl sulfoxide (Sigma) was added. The plates were agitated until the crystals dissolved. Reduced MTT was measured spectrophotometrically at 595 nm in a beam microplate reader (Molecular Devices).
Peripheral blood samples collected into anticoagulant-free tubes were centrifuged and stored at -70℃ prior to testing. An experienced researcher at Severance Hospital performed the assays for serum DKK-1. This individual had no access to patients' clinical information. ELISA (R&D Systems) was performed according to the manufacturer's recommendations. Briefly, 96-well Nunc-Immunomicrotitre plates with a Maxi-Sorp surface (NalgeNunc, Penfield, NY, USA) were coated with the DKK-1 monoclonal antibody supplied with the kit and incubated overnight at 4℃. The reaction was blocked with 1% bovine serum albumin. Sera, diluted with 10% neonatal calf serum, were incubated for 2 h at 37℃. The biotinylated goat anti-human DKK-1 detection antibody was incubated for 2 h at 37℃, followed by streptavidin-horseradish peroxidase for 20 min. Colour development was initiated with 3,3, 5,5-tetramethylbenzidine and hydrogen peroxide followed by 1 M sulphuric acid to stop the reaction. The optical density was measured at 450 nm and referenced to 570 nm on a Synergy 2 multimode platereader (Biotek, Winooski, VT, USA). The DKK-1 concentrations were obtained with a four-parameter logistic curve fitted against a standard curve and multiplied by the dilution factor. When the DKK-1 concentration was less than 0.03 ng/mL (the lower limit of the standard curve), the value was defined as zero. All measurements were performed in triplicate.
AFP concentrations were measured by a UniCel DxI 800 Accessimmunoanalyzer (Beckman Coulter, Inc., Fullerton, CA, USA). Serum DCP concentrations were measured using two different assays: the commercially available EIA (Haicatch PIVKA-II, Eisai Co., Ltd., Tokyo, Japan) and a Lumipulse G1200 autoanalyzer (Fujirebioinc., Tokyo, Japan) according to the manufacturers' instructions. All AFP and DCP measurements were performed in triplicate.
Data are expressed as mean±D, median (range), or n (%) where appropriate. The statistical significance of differences between continuous and categorical variables was examined using the Student's t-test or Mann-Whitney test, and the chi-squared test or Fisher's exact test, when appropriate. Receiver operating characteristics (ROC) curves were constructed to assess sensitivity, specificity, and respective areas under the curves (AUCs) with a 95% confidence interval (CI). The statistical significance between AUC curves were calculated based on DeLong methods. The optimum diagnosis cutoff value was calculated to maximize the sum of sensitivity and specificity, and to minimize overall error. To determine the diagnostic accuracy of DKK-1, AFP, and DCP measurements, we estimated the combined marker functions by binary logistic regression. The function values were used as one marker and subjected to ROC analysis. To assess whether the DKK-1, AFP, and DCP combination marker was better than the individual biomarkers, we created a new variable predicted probability (p) for HCC on the basis of a binary logistic regression equation. The combination formula (DKK-1 plus AFP, DKK-1 plus DCP, and DKK-1 plus AFP plus DCP) indicated simple addition of the value of each component, not dichotomized categorization of each variable (positive vs. negative). The correlation between DKK-1 serum concentrations and clinic-pathological characteristics was analyzed with a Spearman's rank correlation test. All statistical analyses were assessed using the Statistical Package for Social Science (SPSS version 20.0, Armonk, NY, USA). A p-value<0.05 was considered statistically significant.
To determine the DKK-1 expression in HCC cells, reverse transcription-PCR (RT-PCR) and ELISA were performed in Hep3B and Huh7 cells with 293 cells as a negative control. RT-PCR analysis showed high DKK-1 mRNA expression in Hep3B cells and low in 293 cells (Fig. 1A). Consistent with these findings, the secreted DKK-1 concentration in the culture media was high in Hep3B cells, moderate in Huh7 cells, and low in 293 cells (Fig. 1B). Luciferase assays revealed that β-catenin transcriptional activity increased significantly in 293 cells (approximately 2.5-fold) following DKK-1 overexpression. In Hep3B cells, however, the activity remained comparable between mock and DKK-1 overexpression (Fig. 1C).
To investigate the effect of DKK-1 on cell migration and invasion capability, DKK-1 was overexpressed or knock-downed in 293 or Huh7 cells, with low or moderate DKK-1 secretion levels, respectively. In this assay, Huh7 cells with DKK-1 moderate expression, instead of Hep3B with high DKK-1 secretion, were used to prevent the potential masking of knock-down influence due to significantly high DKK-1 secretion in Hep3B cells.
Wound healing assays showed that DKK-1 overexpression efficiently promoted migration of 293 cells (Fig. 2A), whereas DKK-1 knock-down significantly inhibited Huh7 cell migration (Fig. 2B) compared with mock controls. Similarly, invasion assays with Huh7 cells showed that DKK-1 overexpression promoted invasion, whereas DKK-1 knock-down inhibited the invasion (Fig. 2C). MTT assays showed that the DKK-1 knock-down significantly inhibited Huh7 cell growth after 48 h, and the amount of secreted DKK-1 significantly decreased (Fig. 3).
Baseline study population characteristics are described in Supplementary Table 1 (only online). The age and proportion of male participants were statistically similar between HCC patients and controls (p>0.05). However, some factors were significantly higher in HCC patients when compared with controls, including alanine aminotransferase level (mean 54.6 IU/L vs. 32.3 IU/L), proportion of patients with liver cirrhosis (76.4% vs. 43.8%), and HBsAg positivity (85.3% vs. 59.9%) (all p<0.05).
To compare serum DKK-1 levels, the DKK-1 concentration was measured using a commercially available ELISA kit (Fig. 4). Serum DKK-1 concentrations were significantly higher in all HCC patients [patients who underwent surgical resection (n=80) and those who were treated with other modalities (n=137); total n=217; median 1.48 (range 0.03-8.88) ng/mL] than in the three control groups [healthy subjects (n=40), patients with chronic hepatitis (n=46), and those with liver cirrhosis (n=67); total n=153; median 0.85 (range 0.01-2.92); p<0.001]. Among three control groups, DKK-1 levels were statistically similar [median 0.90 (range 0.01-1.91) ng/mL in healthy subjects vs. 0.72 (range 0.05-2.03) ng/mL in chronic hepatitis patients vs. 0.81 (range 0.01-2.92) ng/mL in liver cirrhosis patients; all p>0.05].
We also measured serum AFP and DCP concentration. Similar to the DKK-1 results, AFP and DCP levels were significantly higher in HCC patients than in the control groups (median 39.1 ng/mL vs. 3.3 ng/mL, p=0.011 and 129 mAU/mL vs. 22 mAU/mL, p=0.001 for AFP and DCP, respectively) (Table 1).
To investigate the correlation between serum DKK-1 concentration and HCC progression, patients with HCC were classified according to TNM and BCLC staging. The serum DKK-1 levels of HCC patients according to tumor stage are as shown in Table 1 and Fig. 4. The HCC patients were stratified into early- and advanced-stage HCC [TNM I-II (n=144) vs. TNM III-IV (n=73)]. DKK-1 levels in TNM I-II patients tended to be lower than TNM III-IV patients (median 1.37 ng/mL vs. 1.66 ng/mL; p=0.093). DCP levels were significantly lower (median 61 mAU/mL vs. 1112 mAU/mL; p=0.009), whereas AFP levels were statistically similar (median 30.8 ng/mL vs. 46.4 ng/mL; p=0.516). When the BCLC staging system was applied, DKK-1 levels were significantly lower in BCLC A-B patients (n=146) than in BCLC C-D patients (n=71) (median 1.36 ng/mL vs. 1.73 ng/mL; p=0.014). DCP level was significantly lower (median 64 mAU/mL vs. 579 mAU/mL; p=0.019), whereas AFP was not statistically significant (median 46.35 ng/mL vs. 27.9 ng/mL; p=0.690).
Compared to controls (median 0.90 ng/mL), DKK-1 level in early-stage HCC patients (graded TNM I-II and BCLC A-B) were significantly higher (median 1.37 ng/mL and 1.36 ng/mL, respectively; all p<0.001). Similarly, AFP and DCP levels were significantly different between controls and TNM I-II (p=0.038 and p<0.001) and BCLC A-B stage patients (p=0.046 and p<0.001) (Table 1).
The optimum DKK-1 cutoff value was calculated as 1.01 ng/mL (AUC=0.829; 95% CI, 0.784-0.875; sensitivity, 90.7%; specificity, 62.0%), AFP as 7.5 ng/mL (AUC=0.794; 95% CI, 0.746-0.841; sensitivity, 69.3%; specificity, 87.6%), and DCP as 40.5 mAU/mL (AUC=0.815; 95% CI, 0.771-0.859; sensitivity, 66.5%; specificity, 93.4%). The AFP and DCP cutoff values were similar to our institution's recommended clinical cutoff value (10 ng/mL and 35 mAU/mL, respectively). For ease of interpretation, we adopted 1.00 ng/mL (sensitivity and specificity identical to 1.01 ng/mL), 10 ng/mL (sensitivity, 65.1% and specificity, 89.8%), and 40 mAU/mL (sensitivity and specificity identical to 40.5 mAU/mL) as the DKK-1, AFP, and DCP cutoff values, respectively.
The diagnostic indices and corresponding ROC curves using these cutoff values are shown in Table 2, Supplementary Fig. 1 (only online). In the differential diagnostic accuracy assessment, DKK-1 had a greater AUC for identifying HCC from the controls than AFP and DCP (AUC=0.829 vs. 0.794 and 0.815, respectively) (Table 2, Supplementary Fig. 1A, only online). However, the AUCs of three markers were statistically similar (all p>0.05). Because DKK-1, AFP, and DCP had no statistical correlations (all p>0.05 by Pearson correlation analysis), we combined these tumor markers to determine whether the diagnostic accuracy could be enhanced. The accuracy of DKK-1 plus AFP or DKK-1 plus DCP was significantly higher than DKK-1 alone (AUC=0.901 vs. 0.829, p<0.001 and 0.927 vs. 0.829, p<0.001, respectively). Furthermore, the accuracy of DKK-1 plus AFP plus DCP increased remarkably (AUC=0.952) which was significantly higher than any single marker and DKK-plus AFP and DKK-1 plus DCP (all p<0.001) (Table 2, Supplementary Fig. 1A, only online). When healthy subjects were excluded from the controls, similar findings were observed (Table 2, Supplementary Fig. 1B, only online).
When identifying early-stage HCC patients from the controls, serum DKK-1 showed a higher AUC than AFP and DCP (AUC=0.818 vs. 0.772 and 0.777 for TNM I-II; AUC=0.811 vs. 0.772 and 0.783 for BCLC A-B, respectively) (Supplementary Table 2, only online, Supplementary Fig. 2A and B, only online). When the biomarkers were combined, the triple combination (DKK-1 plus AFP plus DCP) showed the best accuracy (AUC=0.939 for TNM and 0.940 for BCLC, respectively) (Supplementary Table 2, only online, Supplementary Fig. 2A and B, only online). Following exclusion of healthy subjects from the controls, similar findings were observed (Supplementary Table 2, only online, Supplementary Fig. 2C and D, only online).
When correlation analysis was performed between DKK-1 and microscopic HCC characteristics using Spearman's method, DKK-1 showed a significant correlation with necroinflammatory activity (coefficient 0.232, p=0.040) and a borderline statistical correlation to satellite nodules (coefficient 0.203, p=0.072) (Supplementary Table 3, only online). In contrast, microvessel invasion, Edmonson grade, fibrosis, tumor size, and tumor number were not significantly correlated to DKK-1 levels (all p>0.05).
For the 173 patients with a single HCC (≤5 cm), the diagnostic performance of DKK-1 in identifying HCC from controls was better than AFP and DCP (AUC=0.781; 95% CI, 0.708-0.853 vs. AUC=0.744; 95% CI, 0.668-0.820 for AFP and AUC=0.763; 95% CI, 0.691-0.835 for DCP). In addition, the triple marker combination showed the best accuracy (AUC=0.923; 95% CI, 0.886-0.961). When patients with HBV-related chronic liver disease were selected (n=273), the diagnostic performance of DCP in identifying HCC from controls was better than DKK-1 and AFP (AUC=0.814; 95% CI, 0.764-0.864 vs. AUC=0.802; 95% CI, 0.737-0.866 for DKK-1 and AUC=0.772; 95% CI, 0.715-0.828 for AFP). Additionally, the triple marker combination showed the highest accuracy (AUC=0.942; 95% CI, 0.916-0.968).
After exclusion of 40 healthy subjects from the controls, similar findings were observed. DKK-1 was more accurate than AFP and DCP in diagnosing single HCC (≤5 cm) (AUC=0.781; 95% CI, 0.708-0.853 vs. AUC=0.744; 95% CI, 0.668-0.820 for AFP and AUC=0.763; 95% CI, 0.691-0.835 for DCP), whereas DCP was more accurate than DKK-1 and AFP in diagnosing HBV-related HCC (AUC=0.814; 95% CI, 0.764-0.864 vs. AUC=0.802; 95% CI, 0.737-0.866 for DKK-1 and AUC=0.772; 95% CI, 0.715-0.828 for AFP). The triple marker combination showed the best accuracy in diagnosing single HCC (≤5 cm) (AUC=0.896; 95% CI, 0.848-0.944) and HBV-related HCC (AUC=0.942; 95% CI, 0.916-0.968).
The survival of HCC patients has improved due to tremendous advances of surgical techniques and perioperative management; however, the mortality rate is still high. This is primarily due to delayed diagnosis, lack of effective therapies, and frequent recurrence and metastasis. Early HCC detection gives the opportunity to employ curative treatments such as liver transplantation, resection, or local ablative therapy, which are the best way to prolong survival.32 Although serum AFP and ultrasound have been widely used for HCC diagnosis and surveillance, approximately 30-40% of HCC patients are serum AFP-negative and ultrasound detection accuracy for HCC is suboptimal.33 As a result, the widespread use of serum AFP and ultrasound for HCC surveillance has been continuously questioned.
Because current diagnostic methods are insufficient, it is important to identify novel serum biomarkers with high diagnostic accuracy to detect and screen early-stage cancers.33 DKK-1 has recently been recognized as a novel biomarker for HCC.23 Conventionally, DKK-1 is known as a secreted glycoprotein with a crucial role in limb and head development in Xenopus embryos, and as an upstream regulator of T-cell factor/β-catenin that forms a negative feedback loop in the canonical Wnt signaling pathway, favoring appropriate cell growth.1034 In normal tissues DKK-1 is hardly expressed, except in placenta and embryonic tissues.35 Paradoxically, however, DKK-1 has been shown to be secreted from some tumor into blood stream, suggesting that serum DKK-1 may be a useful biomarker for numerous cancers including HCC.36373839 Serum DKK-1 is an advantageous biomarker because it is non-invasiveness, has a simple detection protocol, and is inexpensive to quantify.40 Therefore, integration of serum DKK-1 along with serum AFP and sonographic findings may improve and optimize diagnostic work-ups and surveillance strategies for HCC.39
Recently, Yu, et al.23 found that DKK-1 expression level was associated with β-catenin staining pattern in HCC cell lines, and that DKK-1 overexpression correlated with β-catenin cytoplasmic/nuclear accumulation in clinical HCC samples. In addition, high DKK-1 expression was an independent unfavorable predictor for overall and disease-free survival in HCC patients who underwent surgical resection. Our present results support these observations. Hep3B cells showed high DKK-1 mRNA expression and protein secretion. Additionally, DKK-1 overexpression raised T-cell factor/β-catenin transcriptional activity and promoted migration of 293 cells. DKK-1 overexpression promoted invasion, whereas DKK-1 knock-down inhibited cell growth, migration, protein secretion, and invasion in Huh7 cells. These results indicate that DKK-1 expression has a significant positive association with HCC progression and metastatic potential. Kuang, et al.41 also observed similar influence of DKK-1 on 293 cells. On the other hand, however, DKK-1 did not increase cellular invasiveness of SNU475 cells.42 The authors also found that DKK-1 did not affect invasion-associated proteases such as matrix metalloprotease-2 (MMP-2) that acts on collagen type IV, a major component of the Matrigel matrix and that MMP-2 levels were unaffected by DKK-1 expression in RT-PCR analysis.42 Therefore, it appears that the ability of DKK-1 to inhibit Wnt signaling was abrogated in HCC, most likely due to genetic alterations disrupting the central multi-protein complex that controls β-catenin stability. However, the exact role of DKK-1 played in migration and invasion is unclear, and the molecular mechanism of this controversial phenomenon needs to be further explored.
A recent large-scale multicenter study concluded that the sensitivity and specificity of serum DKK-1 were satisfactory for diagnosing HCC, including early-stage disease and in AFP-negative patients;39 DKK-1 could distinguish HCC from non-malignant chronic hepatitis or cirrhosis, particularly in AFP-positive patients, indicating the potential of DKK-1 as a serum biomarker for HCC. The diagnostic accuracy of DKK-1, AFP, and DKK-1 plus AFP combination was similar to our findings. In our study, the AUC of DKK-1 was higher (AUC 0.825-0.829) than AFP and DCP alone. However, when DCP, a biomarker widely used in Eastern Asia,43 was combined with DKK-1 and AFP together, the diagnostic accuracy of HCC increased significantly with an overall AUC of approximately 0.95 and an AUC of 0.94 for early-stage HCC. However, the calculated DKK-1 cutoff value in our study was much lower (1.01 ng/mL vs. 2.153 ng/mL).39 The exact reason for the DKK-1 cutoff value difference is unclear. It may be due to heterogeneity of carcinogenetic mechanisms because of various etiologies of chronic liver diseases of our study population. Therefore, further study is required to establish an optimal DKK-1 cutoff value.
In our study and in Shen, et al.,39 serum DKK-1 levels were statistically similar among control groups regardless of the presence of cirrhosis. A study by Tung, et al.44 also showed statistically similar serum DKK-1 levels between controls with and without cirrhosis; however, the DKK-1 transcript level was significantly higher in patients with cirrhosis than patients with chronic hepatitis. Further studies are needed to address the discrepancy in DKK-1 serum and transcript levels according to the presence of liver cirrhosis. However, it can be assumed that DKK-1 transcript level may be correlated to the degree of fibrotic burden, which is an important risk factor for HCC development. This is in contrast to serum DKK-1 levels, which are influenced by apparent HCC development rather than high fibrotic burden, an HCC risk factor. In the present study, there was, indeed, no correlation between serum DKK-1 level and fibrosis stage. This might indicate that DKK-1 secretion into the blood stream could be initiated upon hepatocyte tumorigenic transformation, and that hepatocytes with external stressors favoring HCC development, such as high background fibrotic burden, still can control DKK-1 secretion despite the high DKK-1 transcript levels. As seen in our study and others, this phenomenon might enable to diagnose early-stage HCC even in the presence of confounding influences such as liver cirrhosis.39 Preferential early-stage DKK-1 overexpression has already been reported in prostate cancer,45 which further supports this hypothesis. However, there was a significant correlation between DKK-1 level and necroinflammatory activity in our study. The potential influence of necroinflammatory activity should be investigated further and, if necessary, compensated for to enhance the accuracy of serum DKK-1 in diagnosing HCC.
Tao, et al.46 showed that elevated DKK-1 transcript levels in human HCC tissues were significantly correlated with the presence of tumor nodules, Edmondson-Steiner grade, and the presence of vascular invasion. Furthermore, high DKK-1 transcript levels were significantly correlated with venous invasion.44 These data indicate the involvement of DKK-1 overexpression in HCC tissues in HCC invasion and metastasis. In our study, however, serum DKK-1 levels showed only a borderline statistical correlation with satellite nodules (p=0.072) and no correlation with Edmondson-Steiner grade or vascular invasion. This may be due to the limited number of patient samples with available histological information (n=90). When we consider that no macroscopic vascular invasion was identified in any of our study participants prior to surgical resection, the influence of DKK-1 overexpression on vascular invasion might have been underestimated. However, because no significant correlation between serum DKK-1 level and venous invasion was observed by Tao, et al.,46 it should be confirmed whether the serum DKK-1, rather than the DKK-1 transcript levels, reflect the invasive and metastatic potential of HCC. Interestingly, by Tung, et al.44 observed that tumors with DKK-1 expression, established by injection of PLC/PRF/5 HCC cells into mice, showed an increased microvessel density score. Another study showed that DKK-1 mediated endothelial cell activation by suppressing the Wnt/β-catenin pathway.47 In this study, using an in vivo microvascular remodeling animal model, DKK-1 significantly increased vascular density and vessel diameter in adult rats, indicating that DKK-1 may play a role in microvascular remodeling and tumor angiogenesis activation, and possibly accounting for DKK-1-mediated cancer growth promotion in vivo. Similarly, it has been reported that DKK-1 promotes angiogenic and cartilaginous degradation in synovial fibroblasts, accelerating synovial angiogenesis and cartilage destruction. These data indicate that DKK-1 could be a new therapeutic target for future cancer treatment. A molecular-targeted agent similar to sorafenib, which suppresses tumor growth and angiogenesis by inhibiting the Raf/MEK/ERK signaling pathway and receptor tyrosine kinases,48 could potentially be developed. Based on this concept, a very recent study demonstrated that an anti-DKK-1 monoclonal antibody suppressed cell invasion and growth in the lung cancer cell line A549.36 It is highly possible that an anti-DKK-1 monoclonal antibody could prevent HCC metastasis and recurrence.
There are several issues that remain unresolved in our study. First, the HCC diagnosis obtained in our study was not completely based on histopathology. Although histopathology remains the gold standard for HCC diagnosis and evaluation, it is difficult to conduct in large populations. Ultrasonography and radiological examinations remain the most common approach to diagnosing HCC in clinical practices. Furthermore, an in-depth correlation analysis between DKK-1 level, HCC histological features, and surrounding normal liver parenchyma was not feasible. Second, our study participants were all native Korean and HBV was the most common etiology, which may limit the generalization of our findings to other ethnicities and chronic liver disease etiologies. Third, the cross-sectional nature of our study is another limitation. Although DKK-1 expression in HCC tissues has been proved to be a significant independent predictor of long-term prognosis, a prospective study investigating whether serum DKK-1 can also be used as a biomarker for surveillance and prognosis of HCC is needed.
In conclusion, our study suggests that DKK-1 may be a key regulator in HCC progression a promising potential therapeutic target for HCC. We also demonstrated that serum DDK-1 could be a useful marker for diagnosing HCC, especially in combination with AFP and DCP. Further studies are needed to confirm whether surveillance program for HCC in patients with chronic liver diseases should be adjusted according to serum DKK-1 level.
Figures and Tables
Fig. 1
DKK-1 expression in Hep3B, Huh7, and 293 cells. (A) RT-PCR analysis showed high DKK-1 mRNA expression in Hep3B cells, low in 293 cells, and none in the negative control. (B) The DKK-1 concentration secreted in the culture media, determined by ELISA, was high in Hep3B cells, moderate in Huh7 cells, and low in 293 cells. (C) Following DKK-1 transfection, luciferase assays revealed that the β-catenin transcriptional activity was induced in 293 cells. *p<0.01. DKK-1, dickkopf-1.

Fig. 2
DKK-1 promotes cell migration and invasion. (A) DKK-1 overexpression promoted 293 cell migration in wound healing assays. (B) DKK-1 knock-down inhibited Huh7 cell migration in wound healing assays. (C) DKK-1 overexpression promoted Huh7 cell invasion, whereas DKK-1 knock-down inhibited it in invasion assays. *p<0.01. DKK-1, dickkopf-1.
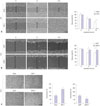
Fig. 3
Knock-down of DKK-1 inhibits cell growth. (A) DKK-1 knock-down in Huh7 cells decreased the amount of secreted DKK-1 after 48 h, determined by ELISA. (B) DKK-1 knock-down inhibited Huh7 cell growth after 48 h, determined by MTT assays. *p<0.01. DKK-1, dickkopf-1.

Fig. 4
Serum DKK-1 concentration. Serum DKK-1 concentrations were significantly higher in HCC patients than those of all the control groups. DKK-1, dickkopf-1; HCC, hepatocellular carcinoma; TNM, Tumor-Node-Metastasis; BCLC, Barcelona Clinic Liver Cancer.

Table 1
DKK-1, AFP, and DCP Levels in the Study Population

Table 2
Diagnostic Accuracy of DKK-1, AFP, and DCP in Diagnosing HCC

DKK-1, dickkopf-1; HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein; DCP, des-gamma-carboxy prothrombin; AUC, area under receiver operating characteristic curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio.
The optimum predicted probabilities of DKK-1, AFP, DCP, and their combination were derived from their respective ROC curves by maximizing the sum of sensitivity and specificity and minimizing the overall error.
ACKNOWLEDGEMENTS
This study was supported by the Liver Cirrhosis Clinical Research Center, in part by a grant from the Korea Healthcare technology R & D project, Ministry of Health and Welfare, Republic of Korea (no. HI10C2020) and in part by a grant from a faculty research grant of Yonsei University College of Medicine for 2011 (6-2011-0145). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001; 35:421–430.

2. Kim SU, Kim do Y, Park JY, Ahn SH, Nah HJ, Chon CY, et al. Hepatocellular carcinoma presenting with bone metastasis: clinical characteristics and prognostic factors. J Cancer Res Clin Oncol. 2008; 134:1377–1384.

4. Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis. 2009; 27:80–92.
5. Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene. 2006; 25:3771–3777.

6. Zucman-Rossi J, Laurent-Puig P. Genetic diversity of hepatocellular carcinomas and its potential impact on targeted therapies. Pharmacogenomics. 2007; 8:997–1003.

7. Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007; 45:1298–1305.
8. Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996; 382:595–601.

9. Wang S, Krinks M, Lin K, Luyten FP, Moos M Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997; 88:757–766.

10. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998; 391:357–362.

11. Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, et al. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999; 274:19465–19472.

13. Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001; 11:951–961.

14. Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002; 417:664–667.

15. González-Sancho JM, Aguilera O, García JM, Pendás-Franco N, Peña C, Cal S, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005; 24:1098–1103.

16. Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene. 2002; 21:878–889.

17. Wirths O, Waha A, Weggen S, Schirmacher P, Kühne T, Goodyer CG, et al. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms' tumors. Lab Invest. 2003; 83:429–434.

18. Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003; 349:2483–2494.

19. Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, et al. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007; 96:646–653.

20. Patil MA, Chua MS, Pan KH, Lin R, Lih CJ, Cheung ST, et al. An integrated data analysis approach to characterize genes highly expressed in hepatocellular carcinoma. Oncogene. 2005; 24:3737–3747.

21. Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008; 68:1451–1461.

22. Lee JD, Yun M, Lee JM, Choi Y, Choi YH, Kim JS, et al. Analysis of gene expression profiles of hepatocellular carcinomas with regard to 18F-fluorodeoxyglucose uptake pattern on positron emission tomography. Eur J Nucl Med Mol Imaging. 2004; 31:1621–1630.

23. Yu B, Yang X, Xu Y, Yao G, Shu H, Lin B, et al. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol. 2009; 50:948–957.

24. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.

25. Chun YH, Kim SU, Park JY, Kim do Y, Han KH, Chon CY, et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer. 2011; 47:2568–2575.

26. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010; 30:61–74.

27. Kim do Y, Kim SU, Ahn SH, Park JY, Lee JM, Park YN, et al. Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig Dis Sci. 2009; 54:1758–1763.

28. Kim SU, Han KH, Nam CM, Park JY, Kim do Y, Chon CY, et al. Natural history of hepatitis B virus-related cirrhotic patients hospitalized to control ascites. J Gastroenterol Hepatol. 2008; 23:1722–1727.

29. Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009; 137:850–855.
30. Fuster J, García-Valdecasas JC, Grande L, Tabet J, Bruix J, Anglada T, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg. 1996; 223:297–302.
31. Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995; 19:1409–1417.
33. Wu W, Yao DF, Yuan YM, Fan JW, Lu XF, Li XH, et al. Combined serum hepatoma-specific alpha-fetoprotein and circulating alpha-fetoprotein-mRNA in diagnosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2006; 5:538–544.
34. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009; 17:9–26.
35. Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999; 238:301–313.

36. Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 2010; 70:5326–5336.

37. Shizhuo W, Tao J, Shulan Z, Bing Z. The expression and significance of Dickkopf-1 in epithelial ovarian carcinoma. Int J Biol Markers. 2009; 24:165–170.

38. Sheng SL, Huang G, Yu B, Qin WX. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009; 55:1656–1664.

39. Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012; 13:817–826.

40. Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Dig Dis Sci. 2010; 55:2744–2755.

41. Kuang HB, Miao CL, Guo WX, Peng S, Cao YJ, Duan EK. Dickkopf-1 enhances migration of HEK293 cell by beta-catenin/E-cadherin degradation. Front Biosci (Landmark Ed). 2009; 14:2212–2220.

42. Kwack MH, Hwang SY, Jang IS, Im SU, Kim JO, Kim MK, et al. Analysis of cellular changes resulting from forced expression of Dickkopf-1 in hepatocellular carcinoma cells. Cancer Res Treat. 2007; 39:30–36.

43. Fujiyama S, Tanaka M, Maeda S, Ashihara H, Hirata R, Tomita K. Tumor markers in early diagnosis, follow-up and management of patients with hepatocellular carcinoma. Oncology. 2002; 62:Suppl 1. 57–63.

44. Tung EK, Mak CK, Fatima S, Lo RC, Zhao H, Zhang C, et al. Clinicopathological and prognostic significance of serum and tissue Dickkopf-1 levels in human hepatocellular carcinoma. Liver Int. 2011; 31:1494–1504.

45. Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate. 2008; 68:1396–1404.

46. Tao YM, Liu Z, Liu HL. Dickkopf-1 (DKK1) promotes invasion and metastasis of hepatocellular carcinoma. Dig Liver Dis. 2013; 45:251–257.

Supplementary Material
Supplementary Fig. 1
ROC curves of DKK-1, AFP, DCP, and their combinations in diagnosing HCC. (A) ROC curves for HCC patients versus all control groups. DKK-1 diagnosed HCC better than AFP and DCP. The combination of three markers (DKK-1, AFP, and DCP) achieved the best accuracy. (B) ROC curves for HCC patients versus all control groups with a risk of HCC. DKK-1 diagnosed HCC better than AFP and DCP. The combination of three markers (DKK-1, AFP, and DCP) achieved the best accuracy. DKK-1, dickkopf-1; AUC, areas under the curves; AFP, alpha-fetoprotein; DCP, des-gamma-carboxy prothrombin; HCC, hepatocellular carcinoma.
Supplementary Fig. 2
ROC curves of DKK-1, AFP, DCP, and their combinations in diagnosing early-stage HCC. (A) ROC curves for patients with early-stage HCC (TNM I-II) versus all control groups. (B) ROC curves for patients with early-stage HCC (TNM I-II) versus all control groups at risk of HCC. (C) ROC curves for patients with early-stage HCC (BCLC A-B) versus all control groups. (D) ROC curves for patients with early-stage HCC (BCLC C-D) versus all control groups at risk of HCC. In all the sub-group analysis, the combination of three markers (DKK-1, AFP, and DCP) achieved the best accuracy. DKK-1, dickkopf-1; AUC, areas under the curves; AFP, alpha-fetoprotein; DCP, des-gamma-carboxy prothrombin; HCC, hepatocellular carcinoma; TNM, Tumor-Node-Metastasis; BCLC, Barcelona Clinic Liver Cancer.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download